1 Introduction
Electrochemical energy storage and conversion devices, including lithium-ion batteries (LIBs), lithium-oxygen batteries, fuel cells, and supercapacitors have been widely applied in the fields of portable electronics, electric automobile, and new power grid systems [1]. Among all battery systems, LIB has been the research focus of electrical energy storage in modern society because of high energy density, in which cathode materials are closely associated with the energy density, security and lifespan of LIB due to their vulnerable structure and a relatively lower specific capacity compared with anode materials [2]. Developing high-capacity cathode materials is extremely urgent to meet the energy density requirements of the next generation LIB. Therefore, LRMOs naturally become the most promising candidate because of their extraordinary specific capacity of over 250 mA h g−1, which is much higher than the current commercialized cathodes [3].
Unfortunately, the actual energy and power density, as well as energy efficiency of LRMOs are still facing challenges because of the serious voltage decay, capacity decay and be relatively poor initial coulomb efficiency. Among these factors, voltage decay in LRMOs is the major obstacle preventing industrial application of LIB. This is because the voltage and specific capacity of the electrodes in a classical LIB determine the energy density [4]. Voltage decay means potential profile and redox peak continuously shifting towards lower voltages during prolong cycling, which inevitably results in a continuous loss of energy density and the difficulty of adapting battery management systems [5]. To defeat this problem, we need to fundamentally understand the voltage decay mechanism of LRMOs.
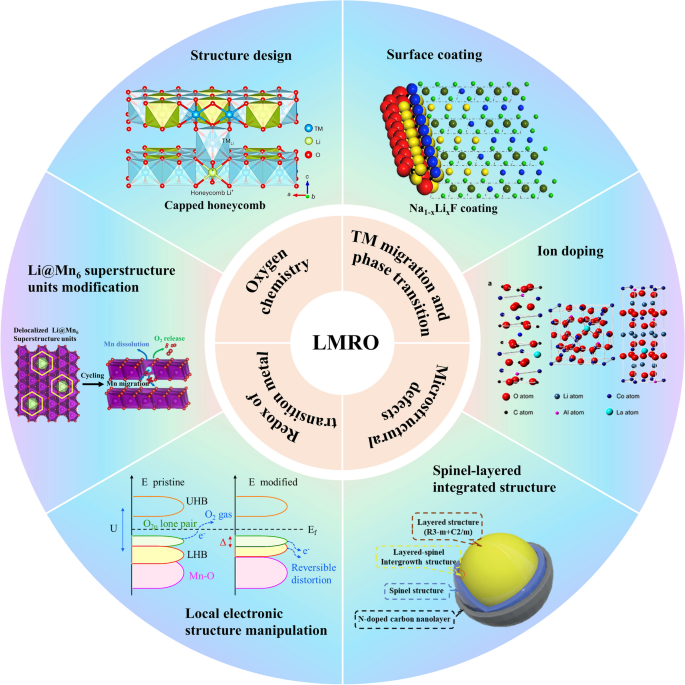
With the advancement of technological techniques, various mechanisms on voltage decay of LRMOs are gradually revealed, such as transition metal (TM) migration, transition metal separation, irreversible phase transitions, the evolution of redox couples, dislocation nucleation, high price theory of manganese [6], oxygen release, the evolution of lattice strain/displacement etc. have been proposed. However, fundamental interplay between these theories has not been fully clarified and some disputes exist, which complicate the study for appropriate strategies. Though enormous efforts have been made to suppress voltage decay, including surface coating, ions doping, Li-poor surface construction [7], surface spinel-layered integrated structure, entropy stabilization [8], component regulation [9], local electronic structure modulation, Li@Mn6 superstructure unit modification [10] and so on, the voltage decay has still not been effectively mitigated. The reason lies in the absent understanding on the relationship between various mechanisms, especially for the origin of voltage decay. It is crucial to elaborate the mechanism and modification strategy systematically and build a complete network of relationships, helping to trace to its source and dissect an effective strategy for mitigating voltage decay.
Herein, we will first introduce the atomic and electronic structure of LRMOs with a full demonstration of its electrochemical behavior along with the corresponding structural degradation. Then the primary voltage decay mechanisms are summarized as structural evolution and oxygen chemistry and discussed in detail, which aims to figure out the correlation of distinct theories. In addition, giving a promising direction for modification strategy, recent efforts made to avoid voltage decay for batteries with high energy density are summarized in the following section. Finally, we put forward a perspective of LRMOs for further development in voltage decay and modification strategies.
2 Structure and electrochemical behavior of LRMOs
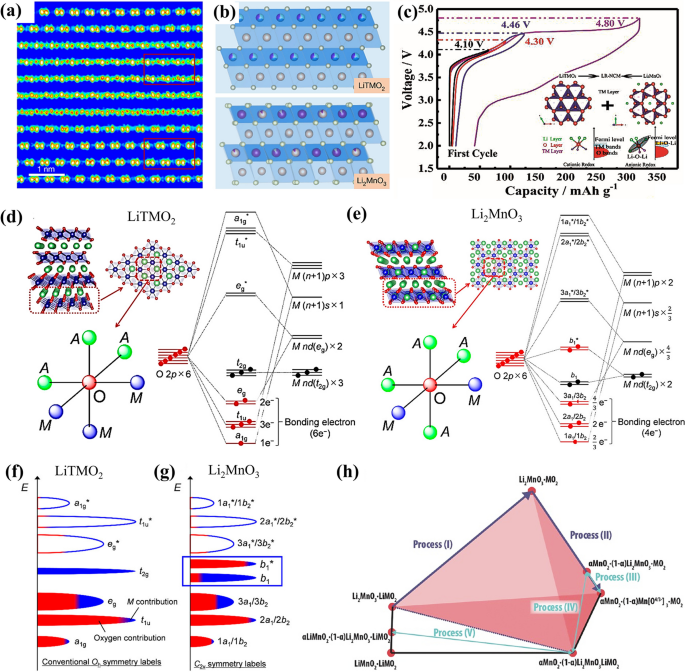
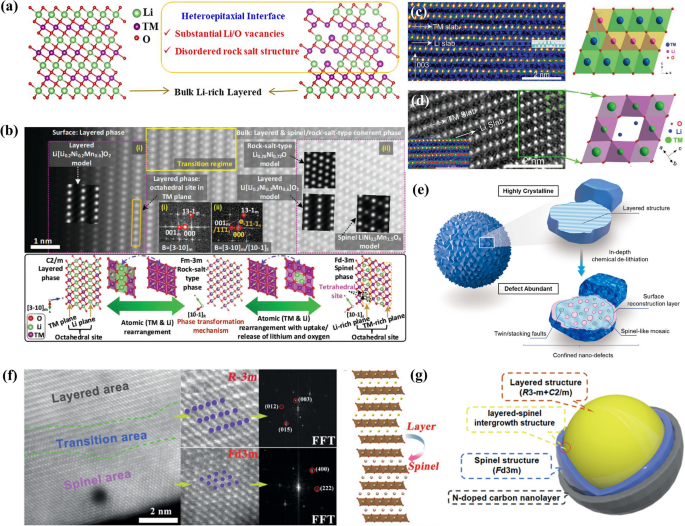
LRMOs typically exhibit higher charge/discharge capacities, typically ≥ 250 mAhg−1 when cycled to higher upper cutoff voltages (≥ 4.5 V) and lower cutoff voltages (≤ 2.5 V), thus delivering the high energy density over 450 Wh kg−1 compared to traditional cathode materials [11]. The cathode is typically considered as a mixture of Li2MnO3, which has a monoclinic C2/m structure, and LiMO2, characterized by a triangular R-3m structure. It is denoted as xLi2MnO3·(1-x)LiTMO2, where TM represents transition metals such as nickel (Ni), manganese (Mn), and cobalt (Co) [12,13,14]. The two structures are closely related, both featuring a cubic close-packed oxygen framework. Within this framework, lithium and transition metals alternate in the octahedral sites, forming an α-NaFeO2 type layered structure. The hexagonal transition metal layer is devoid of lithium, whereas in the monoclinic phase, lithium replaces one-third of the manganese atoms in the transition metal layer, creating a LiMn6 superlattice with a honeycomb pattern [15]. Consequently, Li2MnO3 can be represented as Li[Li1/3Mn2/3]O2. Visually, as depicted in Fig. 1a and b, a standard LRMOs structure is characterized by two distinct arrangements of bright spots. LiTMO2 integrates into the Li2MnO3 lattice in a three-dimensional manner, without distinct phase boundaries, as the two phases are intermixed and share a coherent lattice structure. Traditionally, Li2MnO3 was considered electrochemically inactive in its layered structure, serving as a structural stabilizer due to the challenge of oxidizing Mn4+ to a higher oxidation state. However, when combined with LiTMO2, it becomes active at elevated voltages, exhibiting a plateau above 4.5 V and providing additional capacity [16]. It is this distinctive structure that renders LRMOs a candidate for high-energy–density cathode materials.
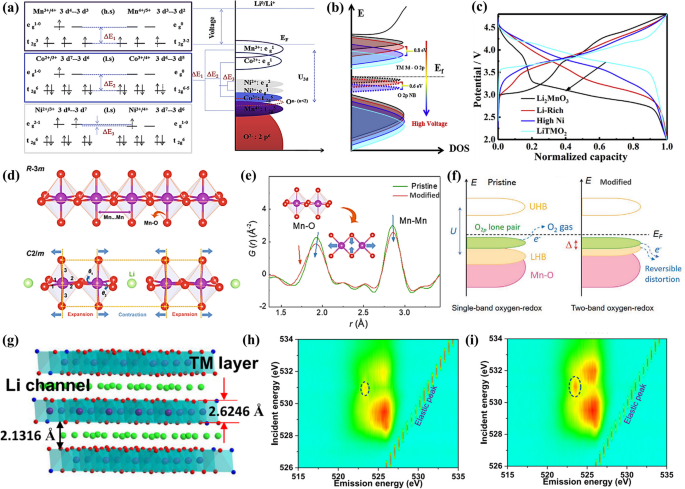
a, b High-resolution transmission electron microscope (TEM) image and schematic structure of LiTMO2 and Li2MnO3 domains. Reproduced with permission [17]. Copyright 2022, Springer Nature. c Charge and discharge curves of LRMOs at 25 mA g−1. Reproduced with permission [18]. Copyright 2020, Wiley–VCH. d, e Molecular orbital energy diagrams and (f, g) schematic band structures of LiTMO2 and Li2MnO3 in LRMOs. Reproduced with permission [19]. Copyright 2017, American Chemical Society. h Compositional phase diagrams. Reproduced with permission [20]. Copyright 2016, Wiley–VCH
The process by which the Li2MnO3 component in LRMOs is activated is intricate. Initially, it was documented that charge compensation is achieved through a Li+/H+ exchange mechanism in a non-aqueous electrolyte [21]. During the charging process, electrolyte oxidation results in the generation of H+ ions at the electrode surface, facilitating an exchange with Li+ within the electrode material. Conversely, during discharge, the electrochemical reduction in the electrolyte consumes H+, prompting its removal from the electrode and substitution by Li+. Further research has indicated that certain structural irregularities, such as Mn3+/oxygen non-stoichiometry and the introduction of Ni impurities [22], can significantly promote the activation of the Li2MnO3 phase [23]. Additionally, the loss of oxygen and O-redox reactions are also implicated in the charge compensation process when the voltage is above 4.5 V (Fig. 1c) [24]. While there is a general agreement that Mn4+ cannot be oxidized to a higher oxidation state within an octahedral oxygen environment, some studies have challenged this view. For instance, Maxwell and colleagues have reported, based on first-principles calculations of the Li1/2MnO3 structure with a face-centered cubic (FCC) oxygen sublattice, that the oxidation of manganese from + 4 to + 7 is indeed possible in LRMOs [6]. To gain a deeper comprehension of the activation mechanism, it is essential to elucidate the further structural distinctions between the LiTMO2 and Li2MnO3 components, which will provide insights into how these materials contribute to the overall electrochemical performance of LRMOs.
Beyond the atomic structure, there are notable variations in the electronic configurations, as illustrated in the inset of Fig. 1c. Within the partial coordination structure of the LiTMO2 component, a single type of O 2p orbital is evident in the Li–O-TM arrangement, where the oxide ions are surrounded by three TM ions and three Li ions [25]. In contrast, the local coordination structure of Li2MnO3 within the LRMOs exhibits two distinct types of O 2p orbitals. The first is the Li–O-TM configuration, similar to that in LiTMO2. The second, unique to Li2MnO3, is the Li–O-Li configuration, where the oxide ions are bonded to four alkali metal ions and two transition metals. The presence of the Li–O-Li configuration results in the emergence of high-energy, pure non-bonding (NB) O 2p states, which are significant in the electronic behavior of the material [18].
According to orbital hybridization theory, the significant energy gap between Li 2s and O 2p orbitals suggests that the lithium atomic orbitals can be disregarded in the analysis. In the structure of LiTMO2, O 2p hybridize with the M (n + 1)s/(n + 1)p to create the primary bonding a1g/t1u and antibonding a1g*/t1u* molecular orbitals, with M representing the transition metal elements in this paragraph (Fig. 1d). Meanwhile, the M nd(eg) orbitals interact with oxygen to establish robust σ bonds, designated as eg and eg*. The nd(t2g) orbitals of M, however, do not participate in bonding with the O 2p orbitals, remaining nonbonding. Given that the Fermi level of LiTMO2 is positioned at the antibonding eg* or nonbonding t2g levels, which are predominantly characterized by the M nd orbitals, the capacity is primarily derived from the transition metal redox reaction, also known as cationic redox (as depicted in Fig. 1f). It should be noted that all O 2p electrons are engaged in a strong bonding state at a substantially lower energy level, making it difficult for them to contribute additional capacity through further oxygen oxidation. In the case of Li2MnO3 within the LRMOs structure, four O 2p orbitals are involved in the formation of σ-type bonding (a1 and b2) and antibonding (a1* and b2*) molecular orbitals with M nd(eg), (n + 1)s, and (n + 1)p. Along the A-O-A axis, where A stands for lithium, two O 2p orbitals and two M nd(t2g) form π-type unbound (b1) and antibonding (b1*) molecular orbitals (as shown in Fig. 1e). The π-type interaction between the occupied O 2p and M nd(t2g) is relatively weak, and the O 2p orbitals near the Fermi level appear to be nonbonding, referred to as nonbonded (NB) O 2p states or O 2p lone pairs (as illustrated in Fig. 1g). This characteristic permits the occurrence of oxygen-redox reactions, which can lead to an increase in capacity.
The distinct local coordination environments within a material are associated with varying redox mechanisms. The Li–O-TM configuration predominantly facilitates cationic redox reactions, whereas the Li–O-Li configuration is more likely to trigger oxygen-redox reactions. This distinction results in notable disparities in the charging and discharging profiles. In contrast to LiTMO2, LRMOs exhibit an extended plateau above 4.46 V during the initial extraction of Li+, signifying the activation of the Li2MnO3 component, which is also the primary cause of structural degradation. During the charging process when the voltage is below 4.5V, the redox reaction involves the transition metals, separating lithium ions from the LiTMO2 layer. Beyond 4.5V, the monoclinic Li2MnO3 becomes active, resulting in the formation of MnO2 and Li2O, with oxygen being released. This process accounts for the irreversible capacity loss during the initial Li+ extraction/insertion cycle. Taking the Li2MnO3·LiNi0.33Co0.33Mn0.33O2 as an example, the Li+ extraction/insertion process involves five distinct steps (Fig. 1h). In the first step, similar to cation redox, the transition metals undergo oxidation and reduction, which facilitates the removal of Li+. This reversible process is represented by Eq. 1. As the charge voltage increases, the Li2MnO3 component becomes activated. In this state, the transition metal d-orbitals and oxygen p-orbitals overlap, forming non-bonding (NB) O 2p states, which can lead to both reversible and irreversible oxygen reactions. The chemical reaction for this process is given by Eq. 2, where [O2−] denotes the resulting oxides such as Li2O or O2, and [O4/3−] refers to the oxygen species in the crystal that can be reversibly reduced to O2− during charge and discharge cycles, as characterized by redox titration, stoichiometric analysis, Extended X-ray Absorption Fine Structure (EXAFS), X-ray Absorption Near Edge Structure (XANES), and other techniques.
Some researchers suggest that irreversible oxygen depletion occurs at the particle surface, while reversible oxygen redox (O2−/O22− or O2−/O2n−) takes place in the bulk, contributing to a portion of the overall capacity [26, 27]. Kun Luo et al. [28] used Li[Li0.2Ni0.2Mn0.6]O2 as the research material, demonstrated that during charging to 4.5 V, each cell releases 0.43 electrons due to the oxidation of O2−, with an additional 0.06 electrons released from lattice oxygen. Consequently, when the charge is further increased to 4.8 V (Process III), the chemical reaction can be described by Eq. 3. In the subsequent discharge processes (IV and V), the reactions are represented by Eqs. 4 and 5, respectively. These equations clearly illustrate the irreversible structural changes that occur during the first Li+ extraction/insertion cycle. After the activation process, which involves full Li+ extraction at 4.8 V, there is a significant gap in the charge/discharge curve, with the discharged capacity predominantly distributed in the lower voltage range. Upon further cycling, the potential profile and redox peaks continuously shift towards lower voltages, a phenomenon referred to as voltage decay.
It is clear that the complex relationship between the local coordination environments and the redox processes within LRMOs plays a pivotal role in their electrochemical performance. The activation of the Li2MnO3 phase, which triggers a series of cationic and oxygen redox reactions, is a significant step in delivering capacity.
3 The structural degradation of LRMOs
With a comprehensive understanding of the structure and electrochemical properties of LRMOs, as previously discussed, there is a consensus that the anionic charge compensation reaction, involving oxygen loss or oxygen redox, is the key to activating the Li2MnO3 component. Though it contributes extra specific capacity, it was recognized as the origin of structural degradation, which encompasses a range of processes, such as the migration of TM ions, phase transformations, and the release of oxygen. The structural deterioration associated with the oxygen loss or redox reaction is highly intricate. Consequently, numerous research endeavors have been dedicated to demystifying this complex phenomenon.
LRMOs experience structural degradation along with a series of problems after the first Li+ extraction/insertion process. Initially, the irreversible oxygen reactions that occur during the first cycle induce material and interfacial structural evolution [29]. The process of Li+ extraction necessitates charge compensation, and for every Li+ ion released, there is a requirement for the removal of 1/4 of an oxygen atom to form Li2O, resulting in oxygen loss and the creation of vacancies. Besides the release of molecular O2, a portion of O2 becomes trapped within the bulk due to the loss of Li+ from the lithium layers and the migration of TM ions, leading to the formation of vacancy cluster defects [30]. The presence of oxygen vacancies can lower the migration free energy, weaken the TM-O bonds, and encourage in-plane and interlayer TM migration, further driving structural transformation. Naoaki Yabuuchi and colleagues performed XRD refinements and estimated that after the initial charge of 0.5Li2MnO3-0.5LiNi1/3Co1/3Mn1/3O2, approximately 7.5% of oxygen vacancies were generated, along with the transfer of about 5% of TM ions from the TM layer to the Li layer [31]. Synchrotron X-ray diffraction (SXRD) and X-ray absorption near edge structure (XANES) spectra data suggest that cation rearrangement takes place during this process, with TM ions migrating into the lithium layer. Further analysis of X-ray absorption spectroscopy (XAS) data indicates that after the departure of oxygen ions, the Fermi energy levels are restructured, and some Mn4+ ions are reduced to Mn3+ during the discharge process. Through visual characterization techniques, Yingchun Lyu and colleagues utilized Scanning transmission electron microscopy (STEM) to identify substantial alterations in both the surface and bulk structures of the material following the initial charge–discharge cycle [32]. It was observed that TM ions had occupied positions in the Li layer on the surface, with no mixing occurring in the bulk phase. Adrien Boulineau and colleagues also detected a minor presence of a spinel-like structure, with TM ions migrating towards the Li layer [33]. This phenomenon indicates that alongside the oxidation and reduction of manganese ions and the irreversible oxygen reaction, the surface of the cathode has undergone a structural reconstruction.
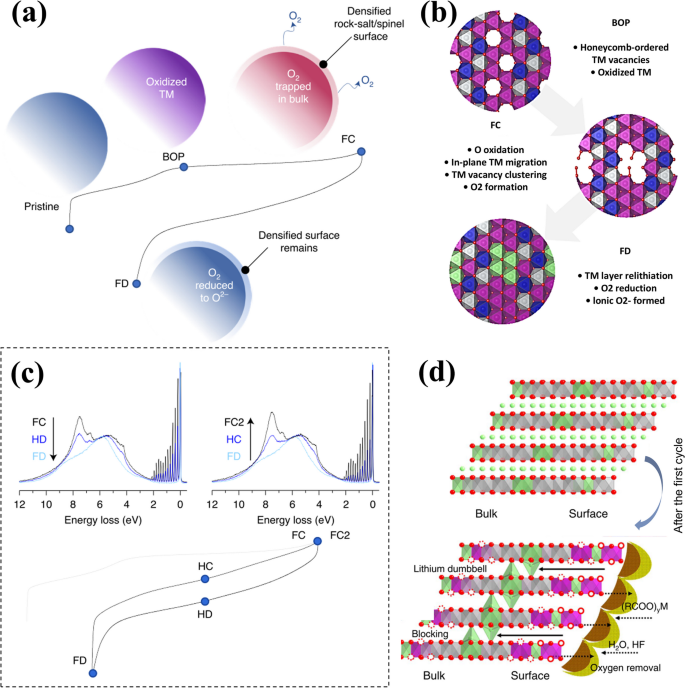
The interaction with the electrolyte will further aggravate the deterioration of the material structure. During the discharge process, a relithiation of the TM layer occurs alongside the reduction of oxygen below 3 V, leading to the formation of O2− and oxygen radicals, as depicted in Fig. 2a and b. The carbonate-based electrolyte solvents, including dimethyl carbonate (DMC), propylene carbonate (PC), and ethylene carbonate (EC), are susceptible to react with these oxygen radicals. These reactions result in the opening of the ring structures of solvents, producing water (H2O), carbon dioxide (CO2), and a range of other carbon-containing compounds. Furthermore, the interaction of oxygen radicals with CO2 can lead to the formation of C2O62−, which subsequently can transform into Li2CO3. This transformation is a significant contributor to the surface side reactions on the electrode. These side reactions deplete the electrochemically active oxygen species and available Li+, thereby contributing to a reduction of reversible capacity. This series of reactions are as follows (Eqs. 6– 9) [34].
a Macroscale changes to the cathode particles. BOP means the beginning of plateau, FC means full charge, FD means full discharge; b Atomic-scale changes to ordering within the TM layer; c HR-RIXS spectra at a 531 eV excitation energy showing the continuous reduction and formation of O2 throughout the first discharge (left) and second charge (FC2 right) (first charge in grey). Reproduced with permission [35]. Copyright 2020, Springer Nature. d The LRMO after the first charge. Reproduced with permission [36]. Copyright 2016, Springer Nature
Overall, the initial charging cycle of LRMOs is marked by the generation of Li and TM vacancies due to oxygen loss in the Li/TM layers. During the subsequent discharge, these vacancies attract TM ions, preventing the reinsertion of Li+ back into the crystal lattice. Concurrently, interfacial reactions at the electrode surface led to the formation of Li2CO3, causing the depletion of some Li ions and a significant irreversible capacity loss of the cathode, which is manifested by low Coulombic efficiency for the first time.
It is clear that during the second charging cycle, the characteristic voltage plateau is absent. After the initial discharge, the ionic interaction in the material weakens significantly, causing the O 2p states at high energy to rise above the average energy of the O 2p states in the original material, where oxygen is coordinated by two TM ions and one Li ion in a nearly non-bonding state. Consequently, these oxygen species are oxidized at a lower potential during the second charge, prior to the oxide ions arranged in the honeycomb structure. This shift in oxidation onset accounts for the absence of a voltage plateau in the subsequent charge cycle. Resonant Inelastic X-ray Scattering (RIXS) data corroborates this observation, showing a substantial production of O2 gas even at potentials as low as 4 V, as illustrated in Fig. 2c.
It is evident that on the second charge, there is a lack of voltage plateau. After the first discharge state, the very weak (nearly bondless) ionic interaction makes the O 2p state in the high energy state higher than the O 2p state of the O2− ion coordinated by two TM and one Li in the original material on average [35]. These ions will be oxidized first in the second charge at a lower potential than the oxide ions in the honeycomb arrangement which explains the lack of voltage plateau in the second charge. The RIXS data supports this because it indicates that a large amount of O2 has been generated even at 4 V (Fig. 2c).
As previously discussed, the breakdown of carbonate-based solvents in the electrolyte leads to the production of H2O. As depicted in Fig. 2d, the progression of this reaction results in the formation of byproducts such as HF, LiF, POF3, and PO43− when H2O interacts with LiPF6 present in the electrolyte. This accumulation of side reaction products on the electrode surface not only augments the electrical resistance but also sequesters active lithium ions, thereby diminishing the overall capacity. Simultaneously, the HF generated can solubilize metal ions from the cathode material, inflicting structural damage. Moreover, the acidic environment established by these reactions can catalyze the disproportionation reaction of Mn3+ ions, a byproduct of the activated Li2MnO3 component. This reaction leads to the loss of active material and the dissolution of manganese, exacerbating the degradation of the cathode and further contributing to the irreversible capacity loss.
As the cycle further progresses, the structural transformations that initially occur at the surface begin to extend into the bulk material. Initially, lithium vacancies form on the surface of the material. Concurrently, nickel ions migrate from the bulk to the surface, while manganese accumulates at the surface, filling the lithium vacancies and creating a tetrahedral restructured layer with a 141 space group. This layer then evolves into a M3O4 (M = Ni, Mn) phase and ultimately transforms into a spinel structure, indicative of a phase transition sequence of C2/m → 141 → M3O4 → spinel [37]. Additionally, Pilgun Oh and colleagues have observed a transition from a layered structure to a spinel-like phase along the [310] crystal band axis of monoclinic structures [38]. This transition results in the formation of a rock-salt phase, which is widely accepted as a contributing factor to voltage decay. This sequence of structural changes further illustrates the complex interplay between surface and bulk processes in LRMOs during cycling, emphasizing the need for strategies to mitigate these effects for improved voltage drop.
4 Voltage decay mechanism
The structural degradation of LRMOs brings many problems, including the irreversible initial capacity loss due to inefficiencies, safety concerns, diminished cycle stability, unsatisfactory rate performance, and notably, the significant voltage lag phenomenon [39]. This voltage decay can significantly undermine the benefits of high specific capacity, as it eventually reduces energy output and efficiency of the battery. Unraveling the comprehensive mechanism behind this substantial voltage decay and devising effective strategies to address it are essential for advancing the development of LIBs with higher operating voltages, which is crucial for enhancing their energy density and performance.
Numerous researchers have dedicated efforts to understanding the voltage decay mechanism in LRMOs. The prevailing theories attributing voltage reduction primarily center around structural evolution and the role of oxygen chemistry. Throughout the cycling process, cationic redox reactions lead to a continuous decrease in the average valence states of the transition metal cations for charge compensation. The ensuing Jahn–Teller (JT) effect can introduce structural strain and encourage the migration of TM, which is thermodynamically favorable. The cumulative and irreversible TM migration can precipitate severe phase transformations. These persistent phase changes can engender visible microstructural defects, which are directly linked to structural degradation and are the primary cause of the observed voltage decay [40]. Oxygen-redox reactions can directly lead to the reduction of transition metals, which in turn promotes the formation of dumbbell-like defects and facilitates the transformation from layered to spinel structures. It is still unclear which mechanism triggers the structural degradation firstly, oxygen chemistry or TM migration. Based on the extensive researches, it is evident that there is an interplay between them, which always appears at the same time. The details of voltage decay mechanism are demonstrated as follows.
4.1 Structural evolution
4.1.1 The evolution of redox couples
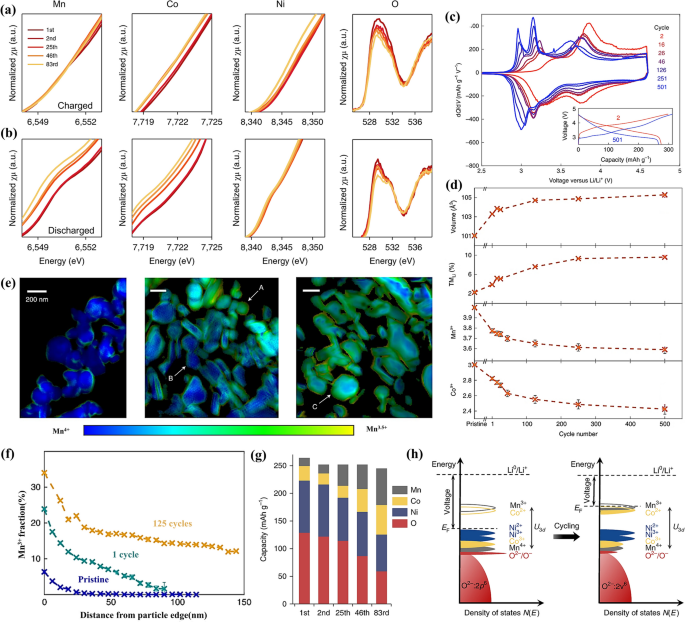
The continuous insertion and extraction of lithium ions during cycling lead to a gradual decrease in the average valence state of various transition metal cations. In addition to the initial redox pairs such Ni2+/Ni3+, Ni3+/Ni4+, the low-voltage redox pairs like Mn3+/Mn4+ are also activated, which directly leads to the voltage attenuation [41], as illustrated in Fig. 3a and b. Ikuma Takahashi and colleagues also conducted research using X-ray diffraction (XRD) and soft X-ray absorption spectroscopy (SXAS) to examine the redox behavior of transition metals in LRMOs [42]. They found that during charge and discharge, Mn and Ni ions are oxidized to Mn3+ and Ni3+, respectively, to compensate for the charge. Mn3+ typically adopts a high-spin state with a large magnetic moment, and the JT effect in MnO2 slabs, which aligns along the elongated axes, results in significant structural distortion [43]. Similarly, Ni3+ with an electronic configuration of t2g6eg1 also exhibits the JT effect, leading to structural distortion that can initiate cation migration [44]. Moreover, Mn3+ is prone to disproportionation, reacting via 2Mn3+ → Mn2+ + Mn4+, which fosters the creation of dumbbell-like defects and facilitates the transition from layered to spinel structures [45]. In contrast, Co3+ does not exhibit the JT effect, suggesting that cobalt ions are likely to remain in the transition metal layer. Consequently, the migration of manganese and nickel ions to the lithium layer during charging and discharging results in the formation of rock-salt-type MnO and NiO at the surface [46]. As the charge–discharge cycles continue, the accumulation of Mn3+ and Ni3+ ions can induce severe phase transformations, negatively impacting the low-potential performance of LRMOs, as depicted in Fig. 3c.
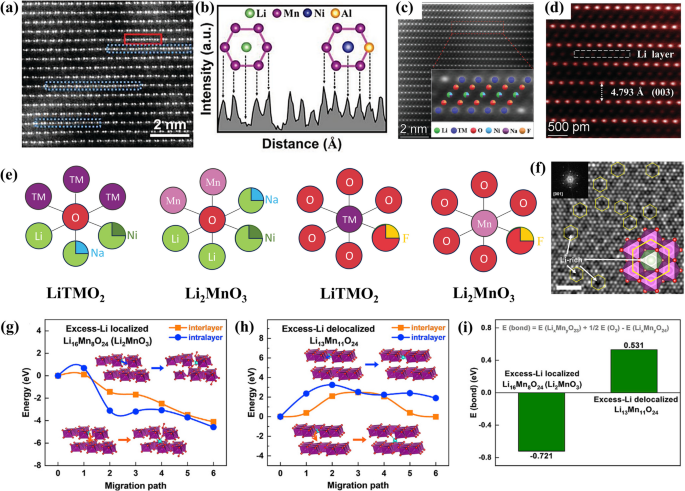
K-edge XAS analysis of manganese, cobalt, nickel and oxygen from LRMO collected after 1, 2, 25, 46 and 83 cycles of (a) charged state and (b) discharged state; Reproduced with permission [41]. Copyright 2018, Springer Nature. c Electrochemical differential capacity plots of LRMOs; d The extent of lattice volume expansion and TM reduction over cycling; e The Mn3+ content as a function of distance from the particle edge; f X-ray ptychography images of the pristine material, after 1 cycle and 125 cycles. Reproduced with permission [47]. Copyright 2021, Springer Nature. g The contribution of each element to discharge capacity under different cycles; h A diagram of an increase in Fermi levels due to changes in the electronic structure. Reproduced with permission [41]. Copyright 2018, Springer Nature
Furthermore, leveraging transmission-based X-ray absorption spectromicroscopy and ptychography, Peter M. Csernica and team delved deeper into the behavior of cation disordering and reduction among primary particles within the cathode material [47]. As cycling progresses, the presence of TM in the lithium layer octahedral sites increases noticeably, from 2 to 10 atomic percent (at%), as illustrated in Fig. 3d. Both Mn and Co undergo gradual reduction from their original Mn4+ and Co3+ states, respectively. The reduction of Mn is particularly notable, initially occurring at the edges of the particles after just one cycle, and then gradually penetrating into the bulk material (beyond 100 nm) over numerous electrochemical cycles. This progression is both visually and quantitatively represented in Figs. 3e and f. These findings reinforce the perspective that the migration of transition metals or structural evolution initiates at the surface of layered lithium oxide (LLO) cathodes during cycling [48, 49]. This insight is crucial for understanding the degradation mechanisms in LRMOs and for developing strategies to enhance their cyclability and voltage stability.
From the perspective of capacity contribution, in the initial cycle, nickel and oxygen in the typical LRMOs, which contains transition metals Ni, Co, and Mn, are the main contributors to capacity generation. However, as cycling progresses, their contributions wane while those of cobalt and manganese steadily increase, as depicted in Fig. 3g. The reduction of transition metals leads to a decrease in their covalency with oxygen, which in turn reduces the degree of oxygen’s involvement in redox reactions and its ability to transfer charge. In pristine LRMO, the Fermi level is positioned above the Ni2+/Ni3+ redox pair. However, as cycling advances and oxygen is lost, the reduction of transition metals, particularly Co and Mn, activates the Co2+/Co3+ and Mn3+/Mn4+ redox pairs. This reduction shifts the Fermi level to a higher energy state, resulting in a decrease in both the operating voltage and the open-circuit voltage (OCV), as shown in Fig. 3h. This shift is a significant factor in the observed voltage decay, highlighting the need for strategies to stabilize the redox-active elements and maintain the structural stability over cycles.
4.1.2 Transition metal migration and phase transition
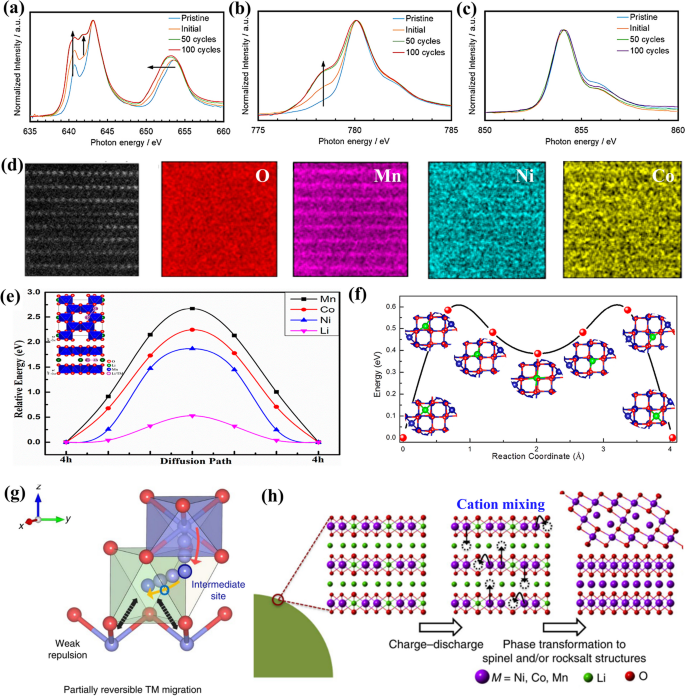
The redox reactions of transition metals are instrumental in driving TM ion migration, which is a key factor in the formation of the spinel phase. During the charging of LRMOs, cations move from the TM layer to the Li layer, creating TMLi-VTM antisite cation-vacancy defect pairs. These phenomena have been experimentally confirmed and characterized using a suite of analytical techniques [50, 51]. For instance, the use of Total Electron Yield (TEY) X-ray absorption near edge structure (XANES) spectra at the Mn L2,3-edge (LII, LIII), Co L3-edge (LIII), and Ni L3-edge (LIII) allows for the examination of the chemical states, thereby providing insights into the occurrence and progression of phase transitions (Fig. 4a-c) [42]. In 2013, Adrien Boulineau and colleagues provided the first atomic-level evidence of manganese-nickel segregation using Scanning transmission electron microscopy (STEM) [52]. In the pristine LRMOs structure, each manganese atom is typically surrounded by three lithium or nickel atoms. When lithium vacancies emerge, manganese can migrate from the surface into the bulk, filling the vacancies within the lithium layer, while nickel migration is impeded by the surrounding manganese atoms. This results in an increasing concentration of nickel at the surface of the electrode during electrochemical cycling, establishing a chemical gradient of Ni/Mn. Pengfei Yan et al. refined the theoretical understanding and introduced the concept of a surface reconstruction layer (SRL), characterized by an outermost layer that is depleted in nickel and enriched in manganese, while the innermost layer is nickel-rich [53]. This layering is closely associated with the movement of nickel from the TM layer to the lithium layer during charge/discharge cycles, followed by the migration of nickel from the crystal bulk to the surface, leading to a gradual depletion of nickel in the crystal lattice. The reduction of manganese ions enhances their mobility, causing manganese to aggregate at the surface. Manganese and nickel ions on the SRL exhibit concentration partitioning.
TEY spectra of (a) Mn LII and LIII, (b) Co LIII, and (c) Ni LIII edges at “Pristine”, “Initial”, 50 cycles, and 100 cycles LRMO electrode. Reproduced with permission [42]. Copyright 2019, American Chemical Society. d Atomic resolution HAADF STEM image of LRMOs in the 4.8 V charged state and corresponding atomic resolution energy-dispersive spectroscopy (EDS) of O, Mn, Ni, and Co element; e Diffusion of energy by Ni, Mn, Co and Li ions in the Li layer; f Migration energy barrier of Co ions in spinel Co3O4 between two neighboring tetrahedral sites. Reproduced with permission [54]. Copyright 2018, American Chemical Society. g Schematic illustrations of cation migration paths. Reproduced with permission [55]. Copyright 2020, Springer Nature. h Crystal structures transition near the surfaces of LRMOs. Reproduced with permission [56]. Copyright 2016, Springer Nature
The preferential migration patterns of transition metal ions (Ni, Mn, and Co) from the TM layer to the Li layer were further investigated by Haijun Yu et al. [54]. Their findings indicate an even distribution of cobalt and nickel across the lithium and TM layers, while manganese remains predominantly in the TM layer (as shown in Fig. 4d). Nickel ions are found to diffuse more readily in the lithium layer compared to cobalt and manganese ions, attributed to a lower energy barrier (as depicted in Fig. 4e). Moreover, the energy barrier for nickel migration from the 4h site in the lithium layer is significantly reduced in the presence of oxygen vacancies generated post-charge [57, 58]. The findings suggest that TM ions exhibit a thermodynamic inclination towards octahedral sites. Once they have migrated to the intermediate tetrahedral sites, the energy barrier for nickel to move from the 4h site in the lithium layer is significantly relduced [59]. As a result, Mn and Ni ions on the 3b site will tend to replace the original Li sites. This leads to an arrangement where Mn and Ni ions alternate on the substituted Li layer, with oxygen on the 6c site and Mn and Ni ions on the original TM layer on the 3a site. The thermodynamic and kinetic stability of the spinel phase TM3O4 were also studied. The results indicates that Co ions have an energy of 0.054 eV/formula unit (fu) more stable in the octahedral sites compared to the tetrahedral sites. There is a migration energy barrier of 0.58 eV for Co ions to move between two adjacent tetrahedral sites, preventing them from occupying octahedral sites, as shown in Fig. 4f. In contrast, for the spinel structure of Ni3O4, the energy of nickel ions at octahedral sites is 0.069 eV/fu lower than at tetrahedral sites [60]. This suggests that the crystal surface of Co3O4 spinel is more stable and irreversible, while Ni3O4 can partly reversibly transform between the spinel and layered structures. As a result, after the initial cycle, the majority of LRMOs have transformed into a distorted manganese-based monoclinic LiTMO2 structure. At the same time, the grain surface has turned into a densified composite structure consisting of rock salt (TMO) and disordered-spinel structure (TM3O4). The insight into the migration dynamics of TM ions and phase transition is crucial for developing strategies to mitigate the structural degradation of LRMOs.
In summary, the electrostatic interactions in LRMO are influenced by the TMO6 octahedra. The edge-sharing between LiO6 and TMO6 octahedra leads to minimal electrostatic repulsion among the cations that are shared along the edges. This low repulsion impedes the reversibility of TM ions, as depicted in Fig. 4g, which contributes to structural phase transitions (Fig. 4h). It is widely accepted that phase transitions, driven by irreversible TM migration, are a primary cause of voltage decay. This understanding is crucial for developing strategies to improve the stability of voltage.
4.1.3 Microstructural defects
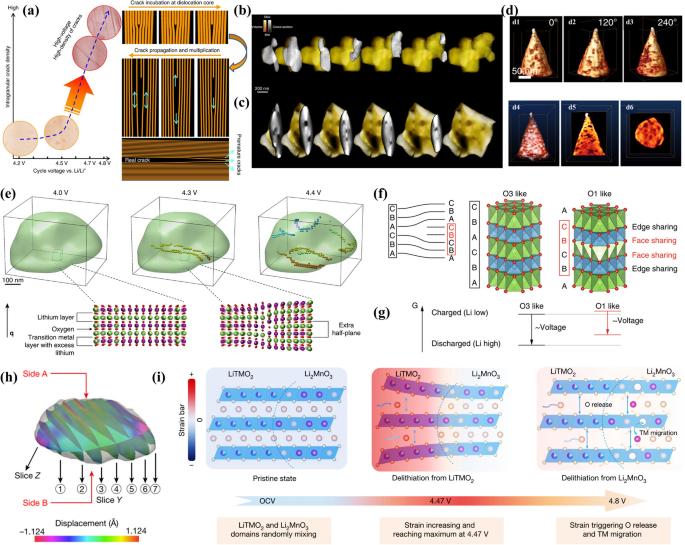
Structural degradation, which is characterized by the formation of microstructural defects, is a direct cause of voltage decay. Research by Ito et al. discovered microcracks in samples subjected to high charging voltages (> 4.5V), suggesting that these cracks could lead to diminished electrochemical performance in later cycles [61]. Zheng et al. reported the presence of sponge-like fragments and structures on the cathode surface after extensive cycling [62]. Haodong Liu and colleagues utilized X-ray and neutron scattering to observe changes in the interlayer spacing (c-lattice parameter) during the charging process, which expanded from 14.25 Å to about 14.40 Å, alongside a contraction of the a and b axes [63]. This phenomenon, known as lattice respiration, is attributed to the stress caused by lithium-ion extraction and TM migration. Initial charging to 4.5V led to substantial volume changes and the disintegration of secondary particles. Cross-sectional thin transmission electron microscopy (CSTT) specimens further elucidated the presence of single secondary particles, multiple single crystalline domains, and nickel-segregation domain boundaries within the cracks [64]. Moreover, each domain was found to contain a “doublet domain” with alternating regions resembling Li2MnO3 and LiTMO2 at the atomic level. The areas rich in Li2MnO3 and the segregated nickel led to a random distribution of domain boundaries, which macroscopically appeared as cracks [65]. The stressed regions within the dislocation core tend to lose lithium and oxygen ions preferentially to alleviate strain, indicating that edge dislocations can act as nucleation sites for crack formation [66]. Below 4.5V, intracrystalline cracking is minimal, but above 4.7V, dislocation-assisted cracking occurs, and the crack density within the grains increases significantly. Over the course of cycling, these cracks spread and proliferate, as depicted in Fig. 5a.
a Intragranular cracking and underlying dislocation-based mechanism. Reproduced with permission [66]. Copyright 2017, Springer Nature. b, c 3D electron tomography reconstruction of pristine LRMO and the state after 15 cycles. Reproduced with permission [41]. Copyright 2018, Springer Nature. d 3D tomography reconstruction showing the spatial distribution of nanovoids. (d1-d3) Surface pits resulting from exposure of interior voids when the sample was sectioned. (d4-d6) Visualization of nanovoid distribution within the grain (The white dots represent nanovoids in d4, while the black dots represent nanovoids in d5, d6). Reproduced with permission [67]. Copyright 2019, Springer Nature. (e) Operando 3D imaging of the dislocation nucleation obtained by in situ BCDI technique; f Schematic of the oxygen layers stacking modification induced by a partial dislocation; g The phenomenological Gibbs free energy diagram. Reproduced with permission [68]. Copyright 2018, Springer Nature; h In situ BCDI images of the 3D LMR particle in the strain field; i Schematic of the correlation of strain generation and O release as well as transition metal migration. Reproduced with permission [17]. Copyright 2022, Springer Nature
Enyuan Hu and colleagues conducted a detailed investigation into the nucleation and development of structural changes within battery materials using Annular dark-field scanning transmission electron microscopy (ADF-STEM) [41]. After 15 cycles, the formation of new large pore groups within the particles was observed, which originated from vacancies created by the loss of oxygen (as depicted in Fig. 5b, c). Tomographic data analysis allowed for a visual representation of the nanovoids’ spatial distribution in three dimensions, confirming their presence within the particles (as shown in Fig. 5d) [67]. By employing in situ Bragg coherent diffractive imaging (BCDI), the researchers were able to clearly observe that the original nanoparticle geometry did not exhibit any irregularities (as illustrated in Fig. 5e). Upon charging to 4.3V, the emergence of two dislocations within the nanoparticles indicated the initiation of dislocation formation due to the charging process. As the charging continued, the dislocation density increased, culminating in the formation of a dislocation network at 4.4V. The degradation of the bulk phase, associated with the development of nanopores, initiates at the particle surface and then gradually extends into the bulk lattice structure as cycling continues. There is a progressive enlargement and multiplication of both partially exposed and fully exposed surface pores. This expansion aids in the propagation of phase structural changes, supports the emergence of further microstructural defects, and consequently, speeds up the rate of voltage decay.
The emergence of dislocations results in the intermingling of O3 and O1 cation ordering sequences within the crystal structure. At the energetically favorable O3 sites, edge-sharing of lithium and TM octahedra takes place, whereas the less favorable O1 sites experience surface sharing (as represented in Fig. 5f). This mixing increases the system’s Gibbs free energy (as shown in Fig. 5g). The energy cost associated with the partial transition from O3 to O1 is heightened at elevated lithium concentrations, due to the increased occupancy of facial shared positions. It is hypothesized that the voltage decay is driven by the free energy difference induced by the presence of O1-like defects.
In a recent study, Tongchao Liu and colleagues meticulously examined the changes in lattice displacement and the evolution of nanostructures using in situ coherent X-ray diffraction imaging that is sensitive on the nanoscale (as shown in Fig. 5h) [17]. They found that the coherent lattice structures of two domains are essential for the generation of lattice strain. The activation of LiTMO2 leads to increased local electrostatic repulsion, which tends to cause the lattice to expand (as depicted in Fig. 5i). Conversely, the lattice expansion of Li2MnO3 domains is partially restricted due to the inactive oxygen redox, which results in significant nanoscale strain and lattice displacement. This strain begins at the particle surface, gradually penetrating into the bulk as delithiation continues, and reaches a peak when the LiTMO2 domains are nearly fully delithiated [69]. The substantial strains caused by this process can significantly destabilize the structure, leading to the decomposition of Li2MnO3 and the release of oxygen. Upon activation of the Li2MnO3 domains, the imposed lattice expansion is naturally released, and the tensile strain is concurrently relaxed. Additionally, the release of oxygen notably reduces the energy barriers for TM migration, leading to an irreversible phase transition [70]. The accumulation of such strain over long-term cycling can negatively impact the material, inevitably leading to structural degradation and rapid electrochemical decay.
4.2 Oxygen chemistry
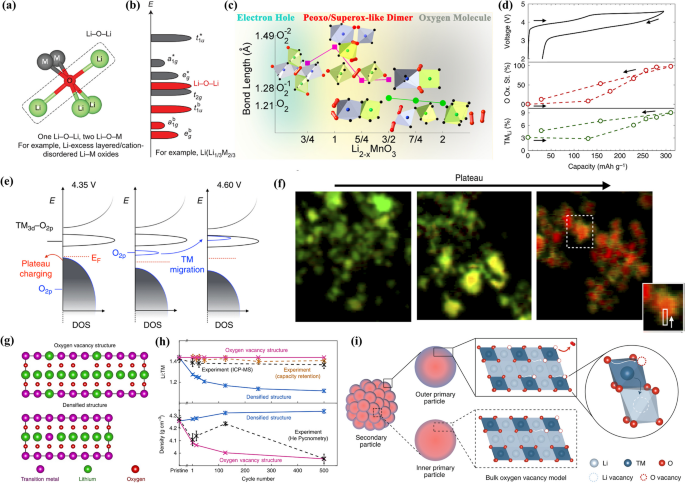
Lattice oxygen is typically considered to compensate for additional charges beyond TM redox by also extracting Li+. In lithium-excess metal oxides, the unique Li–O-Li configuration is pivotal for triggering anionic oxygen redox, which allows for the activation of labile oxygen electrons (as shown in Fig. 6a) [71]. This activation creates unbound oxygen states that shift out of the bonding oxygen manifold, positioning themselves within the TM-influenced complex of eg* and t2g states. This positioning sets the stage for competition between oxygen oxidation and TM oxidation (as depicted in Fig. 6b). In LRMOs, when lithium is extracted, oxygen forms local electron holes that are coordinated by manganese and lithium. Manganese, being in the first row of the transition series, facilitates the localization of electron holes on oxygen atoms, which is crucial for the balance between oxygen loss and oxygen redox chemistry [72]. Research on Li[Li0.2Ni0.2Mn0.6]O2 has indicated that at 4.5V, the oxygen redox potential predominates, accounting for approximately 0.43 electrons per formula unit, whereas the potential for oxygen loss only accounts for 0.06 electrons per formula unit. Beyond 4.5V, the remaining potential is primarily associated with oxygen loss, accounting for about 0.08 electrons per formula unit [28]. Zhenlian Chen and his team elucidated the evolution of oxygen through Density Functional Theory (DFT) calculations combined with the high-throughput Madelung matrix method [73]. Their findings suggest that cationic antisite defects and electron deficiency are the two main limiting factors for anionic oxidation. As the level of delithiation increases, the state of anionic oxidation evolves from electron/hole states, through the formation of peroxo-like O2δ− dimers, to the eventual release of gas-phase oxygen molecules (as illustrated in Fig. 6c). The release of oxygen directly leads to the reduction of transition metals, increasing the involvement of lower-valence TM ions (e.g., Mn3+) in the redox process. These Mn3+ ions are instrumental in the formation of dumbbell defects, characterized by TM ions occupying tetrahedral sites. The formation of such defects is a critical kinetic step in the transformation from layered to spinel structures. Therefore, oxygen loss promotes this layered-to-spinel transformation [45]. The release of oxygen is not only intensified by microstructural defects but also induces the creation of defects, which in turn leads to further oxygen release. In essence, oxygen release and defect formation establish a positive feedback loop [45].
a, b Local atomic coordination of LRMO. Reproduced with permission [71]. Copyright 2016, Springer Nature. c The structural evolution versus the degree of delithiation. Reproduced with permission [73]. Copyright 2019, American Chemical Society. d Plot of the O fractional oxidation state (red) and the migrated TM fraction (green) as a function of capacity; e Schematic of the reorganization of the electronic structure due to TM migration. Reproduced with permission [74]. Copyright 2017, Springer Nature. f Nanoscale distribution of O oxidation for primary particles at different points in the voltage plateau. Reproduced with permission [74]. Copyright 2017, Springer Nature. g Graphical depiction of the two possible structures after 500 cycles, the oxygen vacancy structure (top) and the densification structure (bottom); h ICP-MS, capacity retention and helium pycnometry density measurements (dashed lines). Reproduced with permission [47]. Copyright 2021, Springer Nature. It indicates that the majority of the oxygen release causes the formation of oxygen vacancies; i Generation of bulk oxygen vacancies and oxygen diffusion in Li-rich layered oxides. Reproduced with permission [75]. Copyright 2021, Springer Nature
William E. Gent and colleagues explored the coupling between anion redox and cation migration, discovering that TM migration is inherently linked to the bulk oxygen (O) redox potential [74]. This intrinsic coupling results in a reorganization of the relative potentials of oxygen and TM-O redox couples (as illustrated in Fig. 6d). Ab initio calculations indicate that the coupling effect is driven by significant alterations in the local oxygen coordination environment. These changes cause the O2p state to move to a higher energy region, decreasing the oxygen redox potential by more than 1 V compared to the TM-O hybrid redox potential (as shown in Fig. 6e). This implies that the oxygen redox chemistry is part of a dynamic interplay between structure and redox, described by the reaction (O2− + TM) → (O− + TMmig) + e−. In this dynamic process, irreversible TM migration is correlated with the emergence of spinel-like structures, a phenomenon that is recognized to contribute to voltage decline. Seungjun Myeong et al. have shown that in the charged state, overoxidation of the well-ordered Li2TMO3 phase leads to the generation of numerous reactive oxygen species, which increases the likelihood of hole localization [76]. This situation weakens the covalency of TM-O bonds, particularly Mn–O bonds, leading to lattice oxygen loss and overall irreversible TM-O bond breakage. Specifically, Ni/Co ions that are encircled by reactive oxygen species and oxygen vacancies are highly mobile and migrate towards lithium vacancies, inducing a phase transition from a layered structure to a TM3O4 structure during the cycling process. These characteristics ultimately result in different redox behaviors for Ni/Co and Mn. Ni/Co is inactive while Mn is active, which ultimately leads to the observed voltage decay.
It is now established that both transition metal–oxygen (TM-O) redox and oxygen (O) redox reactions take place throughout the bulk of the primary particles. However, oxygen evolution is confined to high-voltage conditions near the surface regions (as depicted in Fig. 6f) [74]. The limited quantity of O2 generated at the surface leads to surface reconstruction, forming a rock-salt/spinel-like shell. In contrast, O2 within the bulk becomes trapped in vacancy clusters near a TM center and can be re-reduced to O2− upon discharge. Although this re-reduction process is reversible, the order–disorder transition associated with O2 formation, causing the loss of honeycomb ordering and clustering of TM vacancies, is not reversible. Oxygen vacancies, resulting from oxygen release, initially form at the particle surfaces and then infiltrate the bulk lattice, undergoing a condensation process (as shown in Fig. 6g, h) [67]. The presence of oxidized O2n− ions lowers the formation energy and diffusion barrier of oxygen vacancies within the lattice. This reduction significantly facilitates the migration of oxygen vacancies, thereby hastening the degradation of the bulk lattice in LRMOs.
Peter M. Csernica and colleagues conducted a more in-depth investigation of oxygen vacancies using transmission-based X-ray absorption spectromicroscopy and ptychography across various length scales [47]. Their research uncovered nanoscale oxygen deficiencies that gradually permeate from the surface into the bulk of oxide particles (~ 200 nm) over the course of 500 cycles (as illustrated in Fig. 6i). Due to a more extended effective oxygen diffusion length, the inner primary particles release a comparatively smaller amount of oxygen than the outer primary particles. Additionally, the arrangement of primary particles within secondary particles (~ 5 μm) leads to considerable heterogeneity in the extent of oxygen release among different primary particles. This finding underscores the close relationship between oxygen chemistry and structural evolution, both of which contribute to the concurrent voltage attenuation.
In a word, LRMOs exhibit a complex redox behavior involving both transition metals and lattice oxygen. The activation of oxygen redox leads to a competitive environment affecting voltage stability. Research indicates that the intrinsic coupling between TM migration and oxygen redox potential results in significant alterations in the oxygen coordination environment. The progressive penetration of oxygen deficiencies from the surface into the bulk lattice, revealed by advanced X-ray techniques, contributes to the heterogeneity in oxygen release and structural degradation, ultimately impacting voltage retention.
5 Strategies and methods for the prevention of voltage decay
The causes of voltage decay have been clearly investigated, leading to the establishment of several strategies to mitigate this issue. These include preserving lattice oxygen [77], adjusting the local electronic structure [78], optimizing Li@Mn6 superstructure units [79], suppressing electrolyte/electrode reactions [80], and preventing TM ion diffusion to boost structural stability [81]. Engineering approaches such as surface coating [82], ion doping [83], and constructing spinel/layered or Li-poor surfaces have been categorized as solutions [84]. This section provides a systematic review of these strategies and methods aimed at preventing voltage decay, integrating an understanding of the underlying mechanisms with practical tactics. It also offers generalized guidance for designing effective protocols and internal logic to better combat voltage decay, with a focus on cathode-specific strategies.
5.1 Surface coating
To deliver high energy capacity, TM and oxygen ions engage in redox reactions, which can result in oxygen loss and a subsequent significant reduction in both capacity and voltage [85]. This oxygen loss is a consequence of an unstable framework that leads to the release of lattice oxygen, triggered by processes involving oxygen [86]. TM ions are prone to migrate to adjacent lithium layers, causing a phase change in the crystal structure towards spinel and/or rock-salt forms, which causes substantial volume changes and particle cracking. With the cycling extending, these cracks propagate and multiply. To prevent such deterioration, coating was employed, for the structure evolution typically begins from the surface of the particle, and well-designed surface structures can suppress this transition process from the initial stage [87]. Widely used coating materials encompass metal oxides (e.g., Al2O3 [88], TiO2 [89], MgO [90]), fluorides (e.g., LiF [91], AlF3 [92]), phosphates (e.g., AlPO4 [93], LaPO4 [94]), conductive carbon [95], olivine materials (e.g., LiFePO4 [96]), and spinel materials (e.g., Li4Mn5O12 [97, 98]), were used to suppress structural evolution. The mechanism is depicted in Fig. 7a. These coatings serve to insulate the bulk material from the electrolyte, thereby preventing adverse reactions that could lead to bulk degradation, phase collapse, or the emergence of unfavorable phases.
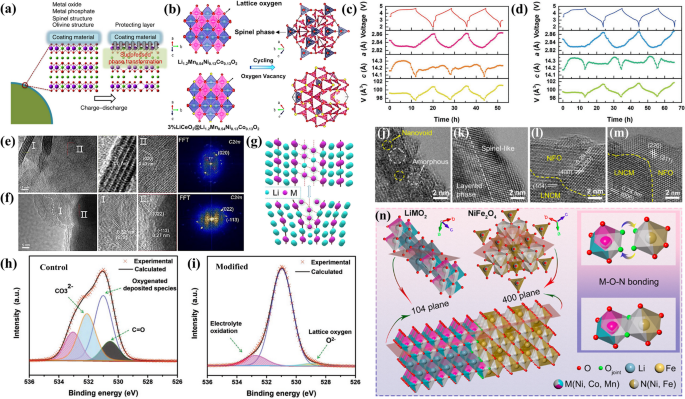
a Conventional surface coating in LRMO. Reproduced with permission [56]. Copyright 2016, Springer Nature. b Schematic diagram of crystal structure evolution before and after the cycle of pristine and 3% LiCeO2 coated LRMO. Reproduced with permission [99]. Copyright 2019, American Chemical Society. Evolution of refined lattice parameters changes during initial three cycles for pristine (c) and modified (d) LRMO. Reproduced with permission [98]. Copyright 2018, Wiley–VCH. TEM images and corresponding FFT of (e) pristine and (f) coated LRMO after 200 cycles; g Structural model of pristine LRMO after 200 cycles; h, i O 1s XPS spectra of pristine and modified LRMO after 200 cycles. Reproduced with permission [100]. Copyright 2019, Wiley–VCH. HRTEM images of local structure for (j, k) pristine and (l, m) NFO coated LRMO after 200 cycles; n Schematic of the heterostructural O-sharing bonding of LNCM (104 plane) and NFO (400 plane). Reproduced with permission [101]. Copyright 2021, American Chemical Society
Liu and colleagues developed a strategy using surface-functionalized LiCeO2-coated materials, which demonstrated a reduced voltage decay of 0.409 V per cycle compared to the 0.75 V per cycle decay of the uncoated sample [99]. This enhanced performance is attributed to the LiCeO2 coating, rich in oxygen vacancies, which shields the bulk material from the electrolyte. The coating prevents direct contact between the bulk and electrolyte, minimizing TM dissolution and SEI layer thickening, which would otherwise increase lithium diffusion resistance [102]. Moreover, the oxygen vacancies in the LiCeO2 coating serve as a buffer, capturing oxygen produced during redox reactions and preventing its irreversible release. This action helps to mitigate phase transitions and further voltage decay (as shown in Fig. 7b) [99]. Beyond single-layer coatings, multi-layer coatings have been implemented to further stabilize the structure. For the proposal of combining extraction/insertion cathodes and electrochemical conversion reaction cathodes, Taolin Zhao et al. designed surface protective layer of LiF/FeF3 [103]. This coating not only inhibits side reactions and provides an electrochemically active surface but also reduces voltage attenuation and the formation of the spinel phase during cycling. Additionally, to address the reliability issues associated with common wet chemistry processes [80, 93], a novel one-step molten salt modification strategy was introduced, resulting in a robust LiF-MgF2-CaF2 coating layer [87]. This protective layer effectively suppresses oxygen release and significantly inhibits TM dissolution, leading to alleviated voltage fading.
An effective surface structure should maintain a uniform crystal lattice at the atomic level with the LRMO phase, ensuring uniform surface formation and integration with the host phase throughout charge–discharge cycles [104]. To enhance compatibility, Zhang et al. developed a 14 nm-thick Li4Mn5O12 spinel coating, in which c-parameter of the pristine fell to 14.08Å and the coated one to 14.18Å (as shown in Fig. 7c, d), indicating that modified cathode has a seamlessly integrated Li4Mn5O12 coated surface [98]. The Li4Mn5O12 spinel epitaxial coating with inherent lattice oxygen stability of protects the fragile layered structure of the bulk, reduces the generation of spinel-like phases, inhibits the subsequent release of lattice oxygen. Consequently, the voltage decay of the treated sample fell to 525 mV after 300 cycles, compared with the original ~ 740 mV [98]. Another kind of material was utilized to suppress the voltage decay. Sijiang Hu and his team applied a hexagonal La0.8Sr0.2MnO3-y (LSM) coating to the LRMO surface. The LSM coating, with its robust Mn–O-M bonds, suppresses oxygen release and mitigates Mn dissolution and phase transformation from layered to spinel. These Mn–O-M bonds also control surface defect and nano-vacancy formation (Fig. 7e-g), stabilizing the LRMO structure during cycling and preventing voltage drop-inducing structural changes. Furthermore, compared to the pristine LRMO, the modified one showed increased activity in surface oxygen redox, providing higher discharge capacity and suppressing voltage decay (as indicated in Fig. 7h-i). The spinel phase, with its closely packed oxygen arrays, is structurally compatible with LRMOs and has been widely used as a coating material [84]. A phase-compatible NiFe2O4 (NFO) layer was employed to transform the nonbonding coordination of surface oxygen into metal–oxygen coordination [101]. After 200 cycles, the NFO-coated LRMO showed reduced TM cation migration and O2 release, curbing structural degradation (as depicted in Fig. 7j-m). It has been reported that bond-length contraction can induce charge transfer from oxygen to metal bonding partners, leading to the generation of vacant O sites [105]. These vacancies are hypothesized to bond and form M–O-N coordination between specific crystal planes (as illustrated in Fig. 7n).
Such a heterostructural O-sharing coating presents three positive features: (1) The O-sharing bonds stabilize the surface lattice oxygens. Thus, irreversible oxygen redox in the form of O2n−/O2 can be suppressed, benefitting to improve voltage stability and enhance first-cycle reversibility. (2) Simultaneously, cation migration into Li sites, which is coupled with oxygen redox, is therefore mitigated. Consequently, they inhibit the layered spinel transition and formation of MLi-VM defects during cycling. (3) Lastly, side reactions such as Mn dissolution can be inhibited through this phase compatible surface coating.
5.2 Ion doping
The release of oxygen from the surface is often accompanied by the diffusion of TM from the surface into the bulk, underscoring the importance of stabilizing the initial structure of both oxygen and TM to restrain voltage decay [106]. While coatings can be effective, they may not address voltage decay originating from bulk processes. Therefore, the incorporation of active metal cations with bulk stabilization properties, through doping, is necessary and has been demonstrated [107]. Ion doping strategies include anion doping (e.g., Cl [81], P [108]), cation doping (e.g., Na [109], W [110]), and co-doping (e.g., Al/Ti [111], Mo/F [112]). The guiding principles for dopant selection typically involve matching ionic radii or valency. The introduction of inert elements is believed to enhance the overlap of the TM(3d/4d/5d)-O(2p) orbital ensemble, thereby strengthening the covalency of TM-O bonds. This reinforcement helps to anchor oxidized lattice oxygen species (e.g., O2n−, On−, TM-On−), slows down cation intermixing, and inhibits TM migration, contributing to the overall stabilization of the material and the mitigation of voltage decay [113].
For bulk anion doping, Wang et al. utilized phosphorus doping to significantly optimize the voltage retention [114]. After doping, Ni2+ ions remain stabilized in their original positions due to the higher electronegativity of pyrophosphate ions compared to O2−, which increases the oxidation state of Ni and inhibits its migration. Concurrently, the strong P-O bonds secure oxygen in place, reducing its release. These factors are pivotal for voltage decay, making anion doping a significant strategy for improving cycle performance and mitigating voltage decay. To shield the surface and decelerate degradation from the surface to the bulk, Zhao et al. employed a gradient polyanion doping strategy [115]. This approach formed a 2 nm spinel-like nano-layer on the surface and a bulk with layered-structured polyanion-doped cathode material, leading to a notable suppression of voltage decay. The presence of phosphate results in abundant nickel ions occupying octahedral positions in the Li layer of the layered structure, and the surface develops a nanoscale spinel-like structure, effectively inhibiting hydrofluoric acid erosion and manganese dissolution. Phosphate doping effectively suppresses TM shift and lessens irreversible capacity and voltage decay in the initial cycle.
As a congener of oxygen, sulfur (S) forms more covalent bonds with TM ions due to its lower electronegativity, enhancing structural reversibility and accelerating electron transfer in Li-rich sulfides [116]. Thus, incorporating S into the lattice of LRMOs is a promising method to modify the TMO6 ligand environment. The regular shift peak in Fig. 8a indicates the reversibility of S(2−n)− (0 < n < 2) redox [116]. The substitution of S atoms for O sites and the formation of TM-S bonds involve a continuous electron loss from the occupied 3p orbitals of S atoms throughout the delithiation process, as evidenced by the continuous decrease in the Density of state (DOS) of the occupied S 3p orbitals (Fig. 8b-d). DFT calculations and Bader charge analysis confirm that S participates in charge compensation throughout the redox process (Fig. 8e). It demonstrates that sulfur anions in the lattice can reversibly engage in the redox process, enhancing overall coordination stability by reducing undesirable oxygen redox. Additionally, S polyanions on the surface form a protective layer for interfacial stability.
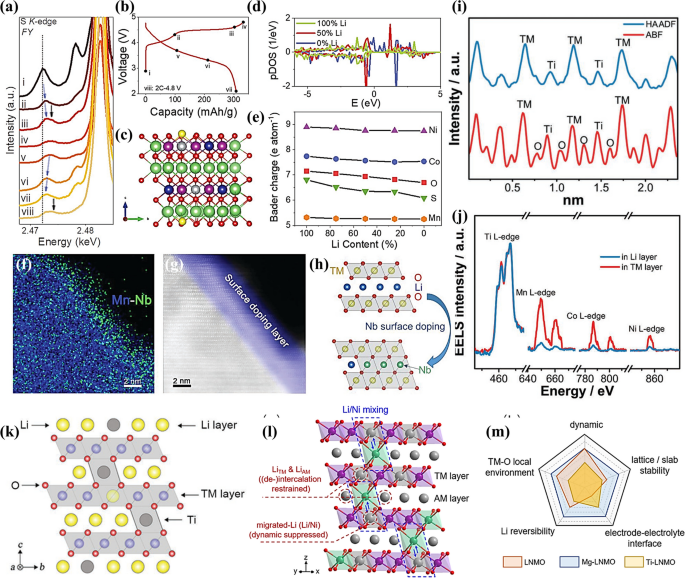
a S K-edge data during charge and discharge stages; b The measured charged and discharged stages. The peak marked by blue arrow is ascribed to the hybridizing redox process of TM and S, while the black arrows mark the new-generated empty states of S atoms; c Calculated atomic structure of S doped LRMO; d Density-of-states of S in the pristine, the half-delithiated and all-delithiated structure; e Bader charge of each element in S doped LRMO during Li removal. Reproduced with permission [116]. Copyright 2022, Wiley–VCH. f The EDS mapping of Mn and Nb; g The surface doping layer; h The schematic process of surface doping and the Nb-enhanced surface structure. Reproduced with permission [117]. Copyright 2018, Wiley–VCH. i Line scan profile of Ti, TM (= Ti, Mn, Ni, and Co), and O along the [421] direction; j The EELS line-scan spectra in the Li layer and TM layer; k The schematic Li-Ti mixed structure. Reproduced with permission [118]. Copyright 2019, Wiley–VCH. l Schematic illustration of the crystal structure used to explain the effects caused by Li/Ni mixing; m The radar map covers five aspects for evaluating cycle performance. Reproduced with permission [119]. Copyright 2023, American Chemical Society
Compared with anion doping, cation doping is a straightforward and widely adopted strategy for enhancing battery performance. Nayak et al. substituted aluminum in the LRMO bulk, stabilizing the voltage at approximately 3.2 V after 100 cycles, a marked improvement over the 3.05 V of control group [107]. Aluminum doping stabilizes voltage attenuation by preventing the phase transition from layered to spinel. Recognizing that TM migration initiates on the surface and extends into the LRMO bulk upon deep delithiation, Liu et al. employed heavy metal doping to the surface for structural stabilization [117]. For instance, doping with niobium (Nb) forms a robust Nb–O bond, passivating surface oxygen and integrating with the doped Nb to form a stable surface structure (Fig. 8f, g). This inhibits the oxidation of bulk particle oxygen, stabilizing tetravalent Mn in the TM layer and preventing phase change, thus maintaining structural integrity and reducing voltage decay (as illustrated in Fig. 8h). To bolster structural tolerance to distortion and curb TM ion migration during (de)lithiation, Shuai Liu et al. introduced a Li-Ti cation mixing strategy [118]. Titanium, heavier than oxygen, occupies the 3a Li position, with a distinct Ti signal in the Li layer, while Mn, Co, and Ni signals are subdued due to electron channel effects (as shown in Fig. 8i-k). The mixed Li-Ti structure prevents layered structure collapse during deep delithiation. In addition, 3d0 electron configuration of Ti allows it to form a strong Ti–O bond, curbing oxygen loss and enhancing structural stability. Furthermore, Ti4+ doping in the Li layer adjusts the role of oxygen in charge compensation to a lower potential, given the closer O2p-TM3d hybridization to the Fermi level, thereby enhancing the overall performance and stability of the LRMO.
To date, many discussions on doping strategies have overlooked the impact of doped or substituted elements on the local scale, particularly within the ordered honeycomb-like structures of Li(Mn)6 and LiNi(Mn)5 in the TM layer, and the octahedral Li(LiO6) in the alkali metal (AM) layers. The specific targets of substitution strategies cannot be merely reduced to “tuning/modifying” the TM-O interactions, such as covalency and bonding environment [77]. Given the unique Li–O-Li configurations in LRMOs, the placement of induced elements can exert varying influences on the lattice. It is essential to understand how these substitutions affect the reversibility of Li+ and anion redox, as well as the local structural stability of TM ions.
Recently, Baodan Zhang and colleagues, using Li1.2Ni0.2Mn0.6O2 as a model, demonstrated a strong correlation between the “degree of disorder” (Li/Ni mixing) and the stability of the interface structure, including the TM-O environment, slab/lattice integrity, and Li+ reversibility [119]. Their findings suggest that the degree of disorder can serve as a reliable indicator for assessing the properties of different materials. Higher degrees of disorder, particularly from Li/Ni mixing, can impede the (de-)intercalation of LiTM and LiAM, introducing additional barriers and obstructing Li+ transfer. Moreover, the dynamics of migrated Li, originating from Li/Ni mixing, can be mitigated (as shown in Fig. 8l). Substitutions with Mg and Ti can polarize the degree of disorder, leading to a stark contrast in global variables from the interface to the bulk structure, such as the TM-O environment, slab/lattice stability, and Li+ reversibility, which in turn results in noticeable differences in electrochemical performance (as depicted in Fig. 8m). The results indicate that the “degree of disorder” is a potent indicator for material modification through element substitution or doping. It represents a promising avenue for doping strategies, potentially offering effective mitigation of voltage decay.
5.3 Spinel-layered integration
In addition to doping and coating strategies, the integration of spinel and layered structures has also been extensively studied. As outlined in our previous work [84], the spinel phase serves several functions: (1) it facilitates initial lithium extraction and full reinsertion, curbing over-oxidation of oxygen; (2) it slows the dissolution of manganese and mitigates structural incompatibility; (3) it offers 3D pathways for lithium ion diffusion; and (4) it inhibits structural distortion. The presence of oxygen vacancies, often associated with the spinel phase, is crucial for preserving lattice oxygen. The spinel-layered integration strategy involves combining the spinel phase with other materials or structures, such as oxygen vacancies, coatings, and doping techniques, to stabilize the structure of LRMOs. These approaches have been significantly validated for their effectiveness in curbing voltage decay by balancing structural evolution with oxygen chemistry.
Oxygen redox reactions in LRMOs initiate from specific Li–O-Li configurations, generating isolated oxygen states. This indicates that both oxygen and lithium activities are crucial in the redox process. Leveraging this understanding, a heteroepitaxial interface with substantial Li/O vacancies, created using molten boric acid (H3BO3), has been proposed (Fig. 9a) [120]. The spinel-like interface with Li/O vacancies enhances electrochemical and structural stability, as well as ionic and electronic conductivity. Weibo Hua and colleagues further explored the formation of the spinel-like structure [121]. Their analysis, as shown in Fig. 9b, demonstrated that layered and spinel-like structurally are compatible phases, sharing a common distorted ccp oxygen lattice, which can coherently grow during thermal treatment. As microwave-heating temperature increases, lithium and oxygen progressively integrate into the spinel host framework derived from the precursor. This process leads to the continuous formation of a rock-salt-type phase (Fm-3m), eventually resulting in the production of a layered phase (C2/m).
a Schematic illustration of heteroepitaxial interface of LRMO. Reproduced with permission [120]. Copyright 2019, Elsevier. b A schematic of the local nature of the structural transformation that occurs and the possible mechanism of atomic rearrangement from the spinel/rock salt type to the layered phase. HAADF-STEM image and crystal model of (c) pristine and (d) spinel-layered integrated sample Reproduced with permission [122]. Copyright 2019, Wiley–VCH. e Diagrams of the bulk modified nanoscale defect-abundant LRMO. Reproduced with permission [123]. Copyright 2019, Elsevier. f HAADF-STEM images and corresponding FFT patterns for spinel-layered integrated LRMO; g Illustration of the structural components. Reproduced with permission [124]. Copyright 2020, Wiley–VCH
Due to structural similarities, the spinel phase can be readily incorporated into LRMOs to reinforce the framework. A synchronous lithiation strategy has been employed to combine doping, surface coating, and spinel phase integration. This approach aims to boost oxygen redox activity and curb the irreversible loss of lattice oxygen, as well as mitigate other LRMOs drawbacks [122]. For instance, nano-Li2SnO3 coating induces spinel phase formation and doping effects, which broaden the lattice spacing, reduce cation mixing, and lower Li+ migration activation energy due to electrostatic interactions (as depicted in Fig. 9c, d). To address the intrinsic voltage attenuation issue, the introduction of spinel-like defects should extend beyond the nanometer scale to penetrate deep into the bulk lattice. Consequently, the synthesized structure resembles a defect-enriched post-cycle hybrids [125]. Following this concept, Haocheng Guo et al. constructed a volume-modified LRMO by directly integrating abundant nanoscale spinel into the lattice through deep chemical de-lithiation (as shown in Fig. 9e) [123]. The significant incorporation of local spinel-like structures, both on the surface and deeply within the volume lattice, alters the intrinsic redox activity. This modification is particularly effective at suppressing voltage decay to an even lower level, exemplified by a minimal decay of 0.281 mV/cycle over a 500-cycle period.
Although oxygen vacancies, spinel phases, and surface coatings individually mitigate capacity fade and voltage decay, a synergistic combination of these strategies is necessary for further structural stabilization. Consequently, Ding et al. employed a urea decomposition strategy to achieve a three-pronged modification, simultaneously creating oxygen vacancies, inducing spinel phase aggregation, and forming nitrogen-doped carbon nano-layers on the surface of LRMO (as shown in Fig. 9f, g) [124]. This comprehensive treatment resulted in a reduced voltage decay rate of 1.09 mV per cycle after 500 cycles, comparing with the untreated 1.45 mV per cycle. The integration of oxygen vacancies and spinel can effectively inhibit irreversible oxygen release and improve the conductivity of lithium ions. Meanwhile, nitrogen-doped carbon layers provide robust protection against electrolyte-induced corrosion while maintaining high electrical conductivity.
It can be concluded that to achieve the effect of spinel-layered integration, adjusting the lithium/oxygen (Li/O) ratio is contributory [18, 126]. In addition to the acid treatment, coating, urea treatment discussed above, facile treatment of NH3·H2O [127], gas–solid treatment including NH3 [128], gaseous sulfur [129], PH3 generated by a pyrolysis reaction of sodium hypophosphite (NaH2PO2) [130], mixed gases of CO and CO2 created by ferrous oxalate dehydrate [131] are also effective.
5.4 Local electronic structure manipulation
The non-bonding O 2p states within the specific Li–O-Li arrangement are crucial for oxygen redox activity. Anionic redox chemistry originates from the relative positioning of energy bands, which are intimately linked to the ligand configurations of the material. The reversibility of the redox behavior is essentially governed by the local ligand geometry of the octahedral layer, affecting how the non-bonding O 2p states and the filled Mn–O bonding states interact. Considering the energy band structure and the anionic redox mechanism, modifying the local electronic structure through component design presents a promising and meaningful avenue for research.
Theoretically, the operating voltage of the electrode is primarily determined by the local electronic structure and the state of the electrons involved in the redox reaction, whether it involves the 3d-electrons of TM ions or the 2p-electrons of anionic oxygen [132]. The 3d orbitals of a TM typically overlap with the 2p orbitals of oxygen, creating TM-O bonds with distinct ionic and covalent characteristics that vary with the metal and anion types and the crystal structure. In LRMO, the TM and oxygen atoms are coordinated in an octahedral configuration (TMO6 octahedra). Within this structure, the energy levels of five 3d orbitals of central TM ions split into two sets: higher energy eg orbitals and lower energy t2g orbitals (as illustrated in Fig. 10a). The continuous reduction of the oxidation state of Ni-Co-Mn ions during cycling, due to oxygen release from LRMO, leads to the formation of lower-voltage Mn3+/4+ and Co2+/3+ redox couples, contributing to voltage fade over cycles.
a The 3d orbitals electronic state of Mn-Co–Ni ions in the TMO6 octahedral structure and the corresponding band structures of LRMO; b Schematic diagram of the electronic structure in different electrode material; c The normalized capacity vs. potential curves of the cathode materials with different local coordination structures at 0.1 C. Reproduced with permission [133]. Copyright 2019, Elsevier. d Schematic of the local ligand orientations for the R-3m and C2/m components; e Low-r PDF patterns of the pristine and modified samples; f Schematic of the single-band oxygen redox process and the two-band oxygen redox process. Reproduced with permission [134]. Copyright 2021, Wiley–VCH. g Crystal model of modified LRMO; The O K-edge RIXS maps of the (h) pristine and (i) modified samples when charged to 4.8 V. Reproduced with permission [135]. Copyright 2022, Springer Nature
Based on these theories, Gang Sun and his colleagues have shown that modulating the local electronic structure by increasing the Ni content in LRMO can improve the operating voltage and suppress voltage decay [133]. In LRMO, a high content of O-Ni2+/3+/Li+ effectively regulates the oxygen coordination environment, increasing the coordination environment of O1 (coordinated with three TMs) and decreasing that of O2 (coordinated with two TMs), thus controlling the local electronic structure and anionic reoxidation (as shown in Fig. 10b). The Ni substitution elevates the oxidation state of TM ions, particularly Mn, to maintain charge balance, reduce the proportion of low-voltage Mn3+/4+ redox couples, and relatively increase the proportion of high-voltage Ni2+/3+/4+ redox couples, which in turn raises the operating voltage (as depicted in Fig. 10c). Through comprehensive physical characterization, electrochemical analysis, and theoretical calculations, the main factors for enhancing the voltage of lithium-rich materials are identified as follows: (1) Increasing the O-Ni2+/3+/Li+ content effectively adjusts the oxygen coordination environment in Li-rich materials, achieving control over the local electronic structure; (2) Ni substitution raises the oxidation state of TM ions, particularly Mn, to maintain charge balance, thus increasing the operating voltage and preventing voltage attenuation; (3) Adjusting the local electronic structure to shift the TM 3d-O 2p band and the non-bonded O 2p band to lower energy levels, thereby improving the redox potential.
It is recognized that irreversible anionic redox reactions predominantly take place on particle surfaces, making surface ligand configuration pivotal for enhancing the reversibility of anionic redox and the overall performance of LRMOs [136]. Yanchen Liu and colleagues introduced a particle-level strategy to adjust surface ligand orientation, thereby preventing the irreversible degradation of LRMOs [134]. A spinel LiCoO2 (LCO) coating layer, compatible with the lattice, was applied to the surface. This modification led to a contraction of the Mn–O bonds pointing towards Li ions from 1.910(5) Å to 1.868(5) Å and a significant reduction in the Mn–O-Mn angle (θ1) within the C2/m structure by 4.52(5)° after the growth of the LCO layer, which in turn triggered an expansion of the Mn–Mn distance along the edge-shared octahedral dimers (as shown in Fig. 10d, e).
The band structure of pristine Li2MnO3 is shaped by the hybridization of Mn 3d and O 2p states, which creates a ligand (Mn–O) bonding band along with two metallic (Mn–O)* antibonding bands: an empty upper Hubbard band (UHB) and a filled lower Hubbard band (LHB), as shown in Fig. 10f. Above these bands lies a fully filled, unhybridized O 2p band, resulting from weakly bonded O 2p lone pair electrons [134]. This isolated oxygen redox process can lead to the formation of unstable On− species that are likely to decoordinate from the lattice, resulting in the irreversible release of O2 gas from the particle surface. In the case of LRMOs with an adjusted surface ligand geometry, the position of the nonbonding O 2p state does not change. However, there is a significant synchronization in the amplitudes of Mn and O near the Fermi level, indicating the formation of a hybridized Mn–O band at this energy level [137]. This hybridization is likely rooted in the LHB, considering the minimal likelihood of interaction between the O 2p lone pairs and Mn atoms. Upon delithiation, electrons from both the O 2p nonbonding band and the (Mn–O)* LHB are made available for redox reactions. The degeneracy of the ground state is alleviated by reversible structural changes, such as the formation of shortened O–O dimers. This dual-band reactive mechanism enhances the reversibility of the anionic redox reaction. The improvement is a result of adjusting the relative positions of the O 2p lone pair bands, which are closely tied to the ligand configuration. By fine-tuning the ligand environment, the redox activity of material is modulated, leading to a more stable and reversible anionic redox process, which is essential for the enhanced structural stability of LRMOs [138].
The determination of band positions is guided by the incorporation of the d-d Coulomb interaction term U and the charge transfer (CT) term Δ, concepts derived from solid-state physics. According to this framework, in many oxides and fluorides, electrons are removed from the filled LHB due to Mott–Hubbard splitting when U is much less than Δ, a phenomenon recognized as cationic redox chemistry. Conversely, in most LRMOs, when U is much greater than Δ, electrons are extracted from the unhybridized O 2p states above the LHB, leading to significant oxygen release, characterized as irreversible oxygen redox [135]. In the intermediate scenario where U is approximately twice Δ, an electron exchange can occur between the nonbonding O 2p state and the (TM-O)* band due to the alignment of the LHB with the unhybridized O 2p bands. To achieve reversible anionic redox and minimize structural damage, it is critical to develop strategies that modulate U and Δ.
To adjust the d-d Coulomb interaction U, strategies involving the creation of oxygen vacancies and reduced manganese in LRMOs have been implemented [135]. Neutron powder diffraction (NPD) indicates an approximate 5% oxygen vacancy, accompanied by an expansion of the Li layer (as shown in Fig. 10g). Both X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) have confirmed the presence of reduced manganese. Incorporating reduced manganese and oxygen vacancies effectively lowers the d-d Coulombic interaction term U, keeping it from greatly surpassing Δ. The enhanced reversibility is evident in the modified sample, where the characteristic fingerprint of oxygen redox activity is more distinctly observed in Fig. 10h, i. This adjustment curbs the release of oxygen and fosters reversible anionic redox reactions [135].
Moreover, a less rigid crystal structure facilitates reversible distortion of the TMO6 octahedra within the TM layers, rather than TM migration caused by a broken rigid octahedral structure, which in turn helps to alleviate voltage decay.
The strategy mainly targets at anion redox chemistry, which is closely related to the structural evolution. Local electronic structure manipulation is affected by both TM 3d and oxygen 2p orbitals. When modulate the TM content in LRMO, the coordination environment of oxygen will change, which can regulate the local electronic structure to reduce voltage decay. In addition, irreversible anionic redox reactions take place mainly on the particle surface, so adjusting the surface ligand configuration is also important for suppressing voltage decay. It can be achieved by regulating the relative band position of the O 2p lone pair state that strongly couples the ligand configuration. What’ more, the band position is determined by the introduction of the d-d Coulomb interaction term U and CT term Δ, combining oxygen vacancies and reduced manganese is a good strategy to regulate U and Δ, and then decrease oxygen release, enhance the reversible anion redox chemistry and reversible distortion of TMO6 octahedra in TM layers, mitigating the voltage decay.
5.5 Li@Mn6 superstructure unit modification
Lattice atoms and their coordination environments constitute the fundamental building blocks of a crystal, which are arranged in a regular pattern defined by a space group, giving rise to the crystal structure. Typically, the bonding interactions and electronic configurations of these structural units dictate the inherent physicochemical properties of the crystal. In LRMOs, the oxygen chemistry stems from the distinctive 180° Li–O-Li configuration attributed to the Li@Mn6 superstructure in the TM layers, where one LiO6 octahedron is surrounded by six MnO6 octahedra. The coordination environment around the oxygen atom is typically characterized by two Li–O-Mn and one Li–O-Li configuration (Mn2Li-O-Li3). Because the hybridized O 2p state in the Li–O-Li bond possesses higher energy, it is more susceptible to oxidation during charging compared to the O 2p state in other configurations, leading to uncontrollable anionic redox activity. During cycling, the migration of Mn ions not only results in the irreversible structural transformation of the Li@Mn6 superstructure and the disruption of the spinel-like phase but also, more critically, leads to the formation of O-Mn0 species within the larger vacancies created by rearrangement. This results in the loss of gaseous oxygen and, consequently, structural degradation over extended cycling periods. Therefore, modulating the Li@Mn6 superstructure units is an effective approach to regulate oxygen redox activity, thereby enhancing the electrochemical performance of LRMOs.
To address these issues, Zhibo Li and colleagues incorporated Al ions to replace Mn sites within the Li@Mn6 superstructure units [79]. These Al ions take the 4g (Mn) positions in the Li@Mn6 units, prompting a partial substitution of Li ions in these units by Ni2+. This substitution results in an evolution of the Li@Mn6 superstructure to (Li/Ni)@(Mn/Al/Li)6, as illustrated in Fig. 11a, b. The modified superstructure units distribute excess Li ions across the TM layers and disrupt the concentrated Li–O-Li configurations, thereby stabilizing the anionic framework and preventing structural degradation during extended cycling. In addition to the Al doping, Na and F were also used to Li@Mn6 superstructure units [78]. The Ni2+ and Na+ ions occupy the Li+ sites of lithium slabs both in LiTMO2 and Li2MnO3, while F substitute the O sites (Fig. 11c-e). It lowers the energy of the TM 3d-O 2p and non-bonding O 2p bands, reducing Li+ diffusion activation energy and increasing the energy required for oxygen vacancy formation. This finally enhances Li+ diffusion kinetics, stabilizes lattice oxygen, and mitigates voltage decay. Moreover, the increased Li–O-Li configuration in the Li2MnO3 phase via increasing the Mn concentration can increase the reversible capacity to offset the negative effect of inactive doping and Li+/Ni2+ intermixing [46].
a Scanning transmission electron microscopy—high angle annular dark field (STEM-HAADF) image of LMNA (Al-doped LRMO) along the c-direction; b The scattering intensity of the ions in the region marked by red rectangle in (a). Reproduced with permission [79]; Copyright 2021, Wiley–VCH. c, d HAADF images along the [010] direction of rhombohedral LiTMO2 (R-3m) in LRMO-NaF; e Schematic illustrations of local atomic coordination around O and TM of LRMO-NaF. Reproduced with permission [78]. Copyright 2021, Wiley–VCH. f HAADF-STEM image along the [001] zone axis (insets showing the corresponding FFT pattern and Li@Mn6 superstructure units highlighted by the yellow hexagons). Scale bars, 1 nm; g Mn migration energy of excess-Li localized Li16Mn8O24 (Li2MnO3) after delithiation (inset showing the Mn interlayer and intralayer migration); h Mn migration energy of excess-Li delocalized Li13Mn11O24 after delithiation; i E(bond) energy of excess-Li localized Li16Mn8O24 (Li2MnO3) and excess-Li delocalized Li13Mn11O24 after delithiation. Reproduced with permission [139]. Copyright 2022, Elsevier
Reports have indicated that the edge-sharing Li@Mn6 superstructure units, resulting from the segregation of excess lithium, can lead to electrochemical irreversibility. This is attributed to the reduced Mn–O covalency and excessive oxygen oxidation during cycling [140]. Consequently, dispersing these Li@Mn6 superstructure units within the TM layers has emerged as a viable and potent strategy, recently proposed by Weiyuan Huang and colleagues [139]. Utilizing an ion-exchange method with a P3-type layered sodic precursor, they prepared LRMO with delocalized Li@Mn6 superstructure units in the TM layers, alongside a pristine deficiency of lithium in the alkali metal (AM) layers (as shown in Fig. 11f). Theoretical study results (as depicted in Fig. 11g, h) demonstrate that the localized excess lithium in the Li2MnO3 structure presents minimal energy barriers and a downhill energy slope for interlayer and intralayer Mn migration. In contrast, the delocalized LRMO with excess-Li exhibits significantly larger barriers, indicating a more stable structure resistant to Mn migration. The E(bond) energy, which is directly associated with the behavior of oxygen loss after full delithiation (as shown in Fig. 11i), clearly indicates that oxygen loss is more probable in the Li2MnO3 with localized excess lithium, and considerably less likely in Li13Mn11O24 where the Li@Mn6 superstructure units are delocalized. The effects of localized and delocalized Li@Mn6 superstructure units can be encapsulated as follows: (1) Delocalized Li@Mn6 superstructure units in the TM layers can elevate the Mn valence from + 3, thereby reducing structural instability caused by Jahn–Teller distortion to a certain extent. (2) The intralayer vacancies resulting from lithium extraction during charging are dispersed, impeding Mn migration both interlayer and intralayer. (3) The oxygen coordination environment remains stable, with regulated oxygen redox activity that suppresses oxygen loss in conjunction with the inhibited Mn migration. The dispersion of Li@Mn6 superstructure units also has a big influence on the oxygen activation [141]. The uniform dispersion of Li@Mn6 superstructure units obtained from the high temperature synthesis can effectively suppress the irreversible oxygen activation. It greatly enhances the reversibility of oxygen redox, thus achieving the satisfied capacity and voltage retentions.
In summary, modifying the Li@Mn6 superstructure units through various methods, such as incorporating Al ions, employing an ion-exchange technique with a P3-type layered sodic precursor, or adjusting the synthesis temperature etc. can effectively fortify the anionic framework and stabilize lattice oxygen. These strategies help to alleviate structural instability stemming from Jahn–Teller distortion. Consequently, by curtailing structural degradation and enhancing the reversibility of oxygen redox over extended cycling, the voltage decay is significantly reduced.
5.6 Structure design
Traditional LRMOs feature a layered O3-type structure with an ABCABC stacking sequence of oxygen atoms. Under high potential, TM ions irreversibly migrate to the Li layer because the LiO6 octahedra only share edges with the MeO6 octahedra (as shown in Fig. 12a) [142]. When lithium ions are extracted, vacancy tetrahedron sites, which are coplanar with the MeO6 octahedra, form in the lithium layer. Trivalent manganese can then migrate to these high-energy tetrahedral sites and subsequently move easily into the adjacent Li octahedral sites. This TM migration is considered a primary cause of voltage decay and is thermodynamically favorable. Reducing TM migration and the associated phase transitions is crucial for enhancing performance. The O2-type phase, with an ABCBA oxygen stacking sequence, has been reported to exhibit minimal voltage decay due to its unique structure. In the O2-type structure, derived from the P2-type by ion exchange, the LiO6 octahedra share both edges and faces with MeO6 octahedra, making the migration of TM ions from intermediate to adjacent Li sites less favorable. The significant electrostatic repulsion between face-shared cations can aid in the return of TM ions during discharge (as depicted in Fig. 12b).
a Schematic illustrations of crystal structures for P2-, O2-, and O3-type layered oxides. F means share a face, E means share edges. Reproduced with permission [142]. Copyright 2014, Wiley–VCH. b Schematic illustrations of cation migration path in O2-type structure; c STXM differential absorbance spectra of the O K edge and Ni and Mn L3 for the first charge and discharge cycle. Each spectrum shows the difference between two designated points in the electrochemical curve; d The O K-edge mRIXS of O2-type LRMO obtained at each point of (c). Reproduced with permission [55]. Copyright 2020, Springer Nature. e HAADF-STEM image of O2/O3- LRMO (green and yellow dotted regions frame oxygen stacking sequences of O3-type ABCABC and O2-type ABCBA, respectively). Reproduced with permission [143]. Copyright 2022, Elsevier. f STEM-HAADF image along the [109] zone axis; g The enlarged area in (f); (h) Schematic diagram of atomic arrangement; HADDF in-line profile acquired along the yellow line (i) and green line (j) in (g); k Sectional view of the structure with TMLi atoms located just next to the honeycomb; l The schematic diagram of the capped-honeycomb local structure. Reproduced with permission [144]. Copyright 2023, Springer Nature
Donggun Eum and colleagues have designed an O2-type structure to minimize voltage hysteresis [55]. Scanning transmission electron microscopy (STEM) measurements clearly visualize the intra-cycle reversible behavior of TM ions. First-principles calculations indicate that the O2 structure restricts the movement of transition metals within the lithium layer and effectively simplifies the migration path for TM ions returning to their original sites. With improved reversibility of TM migration, symmetric anionic redox can also be achieved, as confirmed by Scanning transmission x-ray microscopy (STXM) differential absorbance spectra and O K-edge momentum-resolved inelastic X-ray scattering (mRIXS), where oxygen is oxidized at high potentials and fully reduced during discharge (as shown in Fig. 12c, d). In essence, by altering the stacking order of oxygen in the layered structure, the reversibility of cation migration in LRMOs can be significantly enhanced, leading to a notable reduction in voltage attenuation.
While the O2-type LRMO has achieved structural reversibility and reduced voltage decay, its capacity retention does not match the improvement in voltage retention. A new approach is to combine O3-type and O2-type structures, creating an alternating arrangement of O2 and O3 lattice stacking sequences. STEM observations have identified two distinct oxygen stacking sequences within the Li2MnO3 lattice: the O3-type ABCABC (marked in green) and the O2-type ABCBA (marked in yellow), confirming the formation of a homogeneous O2/O3 hybrid structure (as shown in Fig. 12e) [143]. The homogeneous interweaving of the O2 structure with the O3 structure acts as a structural stabilizer, pinning the O3 lattice and enhancing the overall stability of the hybrid framework. This may be attributed to the lattice mismatch between the O2 and O3 phases, which could induce Mn–O octahedral distortion. This distortion helps tune the energy of the O2p electrons for reversible oxygen redox reactions and stabilizes the honeycomb structure during cycling. The O2/O3 hybrid structure offers several benefits: it alleviates cracks, reduces the formation of the spinel phase after prolonged cycling, and enables reversible anionic redox with less irreversible oxygen loss. These factors contribute to superior cycling performance and suppressed voltage decay.
It has been established that the distinct Li–O-Li configuration within the honeycomb structure of Li2MnO3 plays a significant role in oxygen chemistry, often resulting in irreversible oxygen release and structural degradation [145]. Consequently, effective strategies have been reported to target the Li2MnO3 phase [146]. D. P. Abraham and colleagues discovered a correlation between the dispersion of the Li2MnO3 phase within the LiTMO2 matrix and the TM migration process [147]. Robert A. House and his team showed that controlling the superstructure can help limit voltage hysteresis and fading [148]. Moreover, studies have indicated that a reduced fraction of the Li2MnO3 phase in LRMOs can restrict TM migration and enable reversible anion redox reactions, thereby managing the local electronic structure [149]. Despite these advancements, voltage decay in LRMOs remains a challenge. Modulating the honeycomb structure in O2-type LRMOs is an emerging strategy. Besides the benefit of large electrostatic repulsion between face-shared cations characteristic of the O2-type structure, this approach can also encourage TM ions to occupy the interlayer Li sites in Li2MnO3. This is facilitated by the unique ABBA oxygen stacking sequence in the O2-type structure, which could be instrumental in stabilizing the structure and enhancing the reversibility of anionic redox reactions.
In a recent breakthrough, Dong Luo and colleagues crafted an O2-type LRMO that exhibits minimal voltage decay [144]. In this design, TM ions embedded in the Li layers of Li2MnO3 create a capping effect that reinforces the integrity of honeycomb structure. Within this capped-honeycomb configuration, the LiO6 honeycomb is stabilized by the direct interaction with TM ions, as validated by STEM-HAADF imaging in Fig. 12f-l. An orderly substitution occurs where every three Li ions are replaced by TMLi ions in a repeating unit with the periodicity of [Li2/3Mn1/3], matching that of [Li1/3Mn2/3] in the TM layer. This suggests that TMLi ions are positioned directly above or below the lithium ions within the LiMn6 honeycomb structure. Each TMLi ion is coordinated with three oxygen atoms from the Li octahedron within the LiMn6 honeycomb, aligning the TMLiO6 and the LiO6 honeycomb octahedra in a coplanar arrangement. This pinning effect of TMLi stabilizes the LiO6 honeycomb. As a result, the capped-honeycomb structure remains stable even after high-voltage cycling, preventing TM migration and oxygen release at their inception and showcasing negligible voltage decay during cycling. This innovation underscores that ensuring the stability of the honeycomb structure is an effective approach to addressing voltage decay in LRMOs.
6 Conclusion and perspective
This review aims to provide an intuitive and definitive understanding of the phenomena of voltage decay and to outline the prevalent challenges and solutions associated with LRMOs. For a comprehensive understanding of LRMO, Fig. 13 summarizes the prevailing mechanisms contributing to voltage decay, along with the mainstream strategies employed for mitigation. Table 1 provides a comprehensive data of the effectiveness of diverse modification methods in alleviating voltage decay. In LRMOs, the presence of the Li–O-Li configuration leads to the generation of high-energy, pure non-bonding (NB) O 2p states, which participate in oxygen-redox reactions. This process manifests as an extended voltage plateau above 4.5 V, offering additional high capacity. Known as Li2MnO3 activation, this electrochemical process is typically accompanied by the dissolution of the superlattice and the irreversible emission of oxygen. The activation process is likely to induce the migration of transition metal ions, creating numerous oxygen vacancies, which in turn lowers the discharge voltage. Furthermore, the formation of these vacancies diminishes the energy barrier for TM migration, accelerating the movement of TM ions. The migration of cations from the TM layer to the Li layer results in the formation of TMLi-VTM antisite cation-vacancy defect pairs, which can further trigger the transformation from a layered to a spinel phase. This phase transition is a direct cause of voltage decay, as it continuously reduces the average valence state of the transition metal cations. The atomic-level migration and phase transition lead to microstructural defects due to the accumulation of these defects. Cracks initially form as a result of lattice breathing, followed by incubation and a propagation and multiplication process, which is directly linked to structural degradation. As the cycling progresses, this degradation becomes increasingly severe, leading to the continuous voltage decay characteristic of LRMOs.
Table 1 The effect of different modification methods on mitigating the voltage decay
| Sample | Strategy | Current density (mA g−1) | Voltage range (V) | Cycle number | Voltage decay (mV/cycle) | Ref |
|---|---|---|---|---|---|---|
| Li1.2Ni0.13Fe0.13Mn0.54O2 | LiF-MgF2-CaF2coating | 0.2C | 2.0–4.8 | 120 | 3.1 | [87] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | LiCeO2coating | 1C | 2.0–4.8 | 200 | 2.45 | [99] |
| Li1.17Ni0.17Co0.17Mn0.5O2 | Li-Mg-PO4layer | 1C | 2.0–4.8 | 250 | 2.4 | [150] |
| Li1.2Ni0.2Mn0.6O2 | Li2SnO3coating | 1C | 2.0–4.8 | 200 | 2 | [122] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | LaPO4coating | 1C | 2.0–4.6 | 200 | 1 | [94] |
| Li1.2Ni0.2Mn0.6O2 | Ti-based integrated layer | 1C | 2.0–4.8 | 500 | 0.72 | [151] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | Mg2TiO4coating | 2C | 2.0–4.9 | 700 | 0.62 | [152] |
| Li1.2Mn0.53Ni0.27O1.976Cl0.024 | Cl doping | 250 | 2.0–4.65 | 300 | 1.73 | [81] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | Ta-Mo doping | 1C | 2.0–4.8 | 250 | 1.58 | [113] |
| Li1.2-2xNaxMn0.56Ni0.16Co0.08O2 | Na doping | 0.5 | 2.5–4.6 | 100 | 1.5 | [109] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | Nb doping | 0.1C | 2.0–4.8 | 100 | 1.36 | [117] |
| Li1.2Ti0.26Ni0.18Co0.18Mn0.18O2 | Ti doping | 0.1C | 2.0–4.8 | 100 | 0.9 | [118] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | S doping | 250 | 2.1–4.8 | 100 | 0.88 | [116] |
| Li1.2Ni0.16Mn0.51Al0.05Co0.08O2 | Al doping | 0.1C | 2.0–4.7 | 100 | 0.7 | [107] |
| Li[Li0.2Ni0.133Co0.133Mn0.534]O2 | P doping | 1C | 2.0–4.8 | 500 | 0.48 | [114] |
| Li1.2Mn0.56Ni0.16Co0.08O2 | W doping | 0.5C | 2.5–4.5 | 200 | 0.43 | [110] |
| Li1.13Mn0.517Ni0.256Co0.097O2 | Al/Ti doping | 1C | 2.0–4.8 | 500 | 0.34 | [111] |
| Li1.2Ni0.2Mn0.6O2 | Mg/Ti doping | 0.5C | 2.0–4.8 | 200 | 0.1 | [153] |
| Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 | Spinel-layered integration | 0.1C | 2.0–4.8 | 50 | 5 | [154] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | Spinel-layered integration | 60 | 2.0–4.8 | 225 | 2.2 | [155] |
| Li1.2Ni0.2Mn0.6O2 | Spinel-layered integration | 1C | 2.0–4.8 | 200 | 2 | [122] |
| 0.5Li2MnO3·0.5LiMn0.4Ni0.4Co0.2O2 | Spinel-layered integration | 1C | 2.0–4.8 | 200 | 1.35 | [156] |
| Li1.2Ni0.2Mn0.6O2 | Spinel-layered integration | 1C | 2.0–4.8 | 500 | 1.09 | [124] |
| Li1.143Mn0.544Ni0.136Co0.136O2 | Spinel-layered integration | 1C | 2.0–4.6 | 500 | 0.28 | [123] |
| Li1.08Ni0.34Co0.08Mn0.5O2 | Local electronic structure manipulation | 20 | 2.2–4.6 | 50 | 3 | [149] |
| Li1.2Mn0.6Ni0.2O2-δ | Local electronic structure manipulation | 50 | 2.0–4.8 | 120 | 1.7 | [135] |
| Li1.2Mn0.54Co0.13Ni0.13O2 | Local electronic structure manipulation | 40 | 2.0–4.8 | 70 | 1.43 | [65] |
| Li1.26Ni0.0741Co0.0741Mn0.593O2 | Local electronic structure manipulation | 100 | 2.0–4.8 | 100 | 1.28 | [157] |
| Li1.17Mn0.44Ni0.35Co0.04O2 | Local electronic structure manipulation | 1C | 2.0–4.8 | 300 | 1.09 | [133] |
| Li1.21Ni0.28Mn0.51O2 | Local electronic structure manipulation | 250 | 2.0–4.8 | 300 | 0.45 | [158] |
| Li[Li1/6Mn1/3Ni1/3Sb1/6]O2 | Li@Mn6superstructure unit modification | 20 | 2.0–4.8 | 50 | 5.6 | [141] |
| Li(1.2-y)NayMn0.56Ni0.16Co0.08O(2-z/2)Fz | Li@Mn6superstructure unit modification | 5C | 2.0–4.8 | 200 | 3.5 | [78] |
| Li[Li1/4Mn1/2Ni1/6Al1/12]O2 | Li@Mn6superstructure unit modification | 0.1C | 2.0–4.8 | 100 | 3.5 | [79] |
| Li0.78Mn0.85Ni0.04O2 | Li@Mn6superstructure unit modification | 0.1C | 2.0–4.8 | 100 | 1.1 | [139] |
| Li1+Y[Ni2/(3+3X)Co2/(3+3X)Mn2X/(3+3X)]O2 | Structure design | 0.1C | 2.0–4.8 | 50 | 4.4 | [9] |
| Lix(Li0.2Ni0.2Mn0.6)O2 | Structure design | 5 | 2.0–4.8 | 40 | 3.25 | [55] |
| Li1.25Co0.25Mn0.50O2 | Structure design | 80 | 2.0–4.8 | 50 | 2 | [137] |
| O2/O3-hybrid LRMO | Structure design | 0.5C | 2.0–4.8 | 100 | 1.18 | [143] |
| Li1.16Mn0.54Ni0.21Co0.08O2 | Structure design | 1C | 2.0–4.6 | 200 | 0.8 | [159] |
| Li1.1(Ni0.21Mn0.65Al0.04)O2 | Structure design | 1/3C | 2.0–4.7 | 50 | 0.02 | [144] |
Evidence suggests that the migration of TM and the release of oxygen in LRMOs are initiated at the particle surface, propelled by surface-interface reactions. Surface coatings are effective in partially mitigating these issues by preventing direct contact between the active material and the electrolyte, thereby reducing oxygen release, phase transitions, and crack propagation. While physical coatings provide a protective barrier, ion doping offers a more intrinsic approach by altering the TM(3d/4d/5d)-O(2p) band overlap. This modification anchors the oxidized lattice oxygen, slows cation mixing, and curbs TM migration. The spinel-layered integration strategy further enhances stability by incorporating elements such as oxygen vacancies, doping, and coatings. Additionally, the oxygen redox activity in LRMOs primarily stems from the non-bonding O 2p states within the specific Li–O-Li configuration. Adjusting the local electronic structure and the Li@Mn6 superstructure can improve the reversibility of oxygen redox and structural stability. These adjustments shift the TM 3d-O 2p bands and the nonbonding O 2p band to lower energy levels, enhancing the reversibility of anionic redox and promoting the reversible distortion of TMO6 octahedra in the TM layers. Among the various strategies, the O2-type structure stands out as particularly effective in reducing voltage decay due to its unique oxygen sequence (ABCBA), which generates significant electrostatic repulsion between face-shared cations. This repulsion aids in the return of TM ions and supports reversible anionic redox. Furthermore, other techniques such as concentration gradient tailoring [159, 160], entropy stabilization [8], cation disorder enhancement [161, 162], and single-crystal policies [163] also contribute to alleviating voltage decay.
It should be noted that the strategies mentioned have not successfully addressed the issue of voltage decay. The mechanisms proposed are speculative and derived from the analysis of LRMOs experiencing voltage decline. This could lead to biased or incomplete conclusions. Very recently, an O2-type LRMOs with capped-honeycomb structure is reported [135]. This capped-honeycomb LRMO presents a negligible voltage decay because its superlattice can maintain stable during cycling. Coincidentally, prior to this, Amine et al. have just claimed that doping and coating cannot eliminate voltage decay of LRMOs, and suggested voltage decay origins from the decomposition of Li2MnO3 honeycomb structure caused by lattice strain/displacement [17]. This viewpoint is well matched by the capped-honeycomb LRMOs which is the only effective strategy to solve the voltage decay problem. Therefore, the instable Li2MnO3 honeycomb structure should be the source of voltage decay. Based on this theory, the above various mechanisms can basically be compatible. The decomposition of Li2MnO3 honeycomb structure inevitably leads to irreversible oxygen release and TM migration, and the surface-interface reactions can accelerate the decomposition process. Herein, surface coating, ion doping and spinel-layered integration etc. cannot effectively reduce voltage decay, but it can enhance the stability of the voltage to some extent.
In the future, there are two possible solutions to solve the voltage decay problem. The one is to strengthen the honeycomb structure to ensure that it can remain stable during the cycle, which has been confirmed by the recent report of capped-honeycomb LRMOs. And the other is to construct low-strain or zero-strain LRMOs to remove the incentive to decompose the honeycomb structure. We hope this review can provide some help to the development of LRMOs with stable voltage.